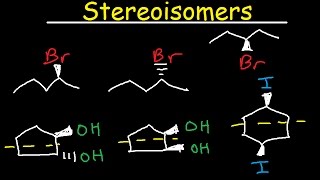
Structural (constitutional) isomers | Structure and bonding | Organic chemistry | Khan Academy
ฝัง
- เผยแพร่เมื่อ 28 ก.พ. 2015
- How to draw structural isomers using bond-line structures.
Watch the next lesson: www.khanacademy.org/science/o...
Missed the previous lesson? www.khanacademy.org/science/o...
Organic Chemistry on Khan Academy: Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Let's make some beautiful organic molecules!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s Organic Chemistry channel: / channel
Subscribe to Khan Academy: th-cam.com/users/subscription_...




![[UNCUT] ฝันรักห้วงนิทรา | EP.9 (3/4)](http://i.ytimg.com/vi/ikd1tEhXbp8/mqdefault.jpg)



Your videos are great, making a tough subject easily understandly!!
You make organic easy ! thank you
Cool I subscribed and liked and even downloaded khan Academy my dad suggested me this app it is the worlds best study app
this was incredibly helpful thank you so much
This saved my life. Thank you!
Thank you! Great explanation!
what about when there is multiple heteroatoms?
i have a the formula C4H8O
and one of the isomers is cyclic.
how do i find cyclic isomers?
thank you for this video, you're better than my teacher but it would have been better if you had named every molecule
Thank you so much! 😊
You made a whole high school topic easy in 10 minutes
Can you PLEASE make a video about isomers of alkenes
Was hoping for help figuring out number of isomers at speed, with tips such as looking at symmetry in the molecule? Good beginners intro though.
Thaaaank you
Why can the 3rd isomer for C3H8O be broken by the carbon chain by an oxygen, but it wouldn't work for something such as C3H7Br where it is deemed a new molecule?
does it matter which end you use on the last example as long as you only use one?
why can't you put 2 carbons onto the middle carbon in the three carbon chain? (first example).
If you look carefully the X chain is just a 3 carbon main chain with 2 carbons attached to the central carbon.
This video is more better than my chemistry class
Arsha Anil more better?
I am confusion. Why do you put lone pairs on the oxygen for the second example?
kya koi isomerism ka koi formula hai jisse ye aasan ho jaye
Can anyone tell me difference between structural isomer and position isomer
Position isomer is a type of structural isomer. Position isomer is the change of the position of functional group for example if the functional group was attached to carbon 2 and there's another one which has the functional grp attached to carbon 3 then they are positional isomers
Isn't the molecule shown at the end of video a ketone, for it has two alkyl groups at the ends of oxygen?
Zahidullah Noori ketone needs a carbonyl group (C = O)
alisharo58 Thanks!
Zahidullah Noori of course :) this stuff is hard! gotta help when able to
how do you know how many hydrogens you add to each carbons?
Carbon has only 4 bonds it can make. Therefore no more than 4 bonds can be attached to it at any time. (So max number of 4 hydrogen could be attached to the carbon). Hope this helps. :)
You ask God
@@joeypadua867 💀💀💀
Dumb question I'm sure but... is there an explanation for why an alkyl can be attached to the end carbons? I'd like to know the explanation
I need to know this as well.. any one ? lol a year later..
dude your audio is too low compared to other khan academy videos
Why the word constitutional used. What that word actually mean
So there's no formula to determine how many isomers?? 🤔
We just have to draw them??
this isn't khan
So there's no formula to determine how many isomers?? 🤔
We just have to draw them??
somewhere i saw an algorithm to calculate the isomers count but it was complicated, and im not sure if that was right, so i would say theres no formula to that