Meeting New Challenges in Toxicological Risk Assessment: Pending Updates to ISO 10993-17
ฝัง
- เผยแพร่เมื่อ 23 ต.ค. 2023
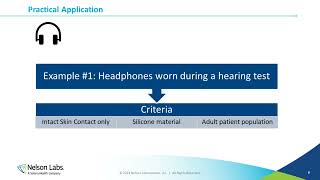
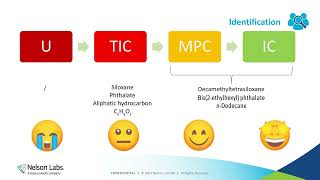
- This presentation will provide both a summary review and a deep dive into some of the key changes that will soon take effect with the planned 2023 release of the revised international standard for toxicological risk assessment of medical devices, ISO 10993-17. Over 20 years have elapsed since the issuance of the original guideline, and over the past 6 years a concerted effort has been underway to revise and update nearly every aspect of the technical requirements in this standard. The talk will focus mostly on the most important key aspects of the upcoming changes to the standard, including:
• Toxicological Screening Limit (TSL): Reducing the number of chemicals to evaluate
• Methodology Details for Estimated Exposure Dose (EED)
• Methodology for Application of the Threshold of Toxicological Concern (TTC)
• Deriving a Tolerable Intake for Non-Cancer Endpoints: Normative Requirements
• Deriving a Tolerable Intake for Carcinogenic Endpoint: Normative Requirements
• Using Release Kinetics Data to Assess Acute and Sub-chronic Exposure Intervals in Conjunction with Relevant Toxicological Studies - วิทยาศาสตร์และเทคโนโลยี




![[UNCUT]”หมอปลา และทนายแก้ว” ลากไส้ขบวนการ เด็กเชื่อมจิต จ่อเอาผิดทั้งครอบครัว I คนดังนั่งเคลียร์](http://i.ytimg.com/vi/9zXrAIVBQFo/mqdefault.jpg)

