Resonance Structures, Basic Introduction - How To Draw The Resonance Hybrid, Chemistry
ฝัง
- เผยแพร่เมื่อ 23 ส.ค. 2024
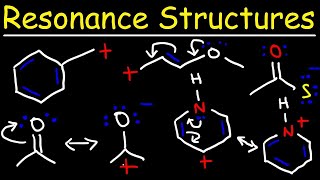
- This general chemistry video tutorial provides a basic introduction into resonance structures. It explains how to draw the resonance structures using curved arrow notation and how to draw the resonance hybrid as well with all of the lone pairs, bonds, and partial charges. This video contains 2 examples / practice problems.
How To Draw Lewis Structures:
• How To Draw Lewis Stru...
VSEPR Theory:
• VSEPR Theory - Basic I...
Molecular Geometry:
• Molecular Geometry & V...
Lewis Dot Structures:
• Lewis Dot Structures -...
Lewis Structures of Ionic Compounds:
• How To Draw The Lewis ...
_________________________________
Octet Rule Exceptions:
• Exceptions To The Octe...
Resonance Structures:
• Resonance Structures, ...
Polar and Nonpolar Molecules:
• Polar and Nonpolar Mol...
Formal Charge Calculations:
• How To Calculate The F...
Lewis Structures - Mega Review:
• Lewis Structures, Intr...
________________________________
Hybridization of Atomic Orbitals:
• Hybridization of Atomi...
Molecular Orbital Theory:
• Molecular Orbital Theo...
Dipole Dipole Forces of Attraction:
• Dipole Dipole Forces o...
Hydrogen Bonding:
• Hydrogen Bonds In Wate...
Unit Cell Chemistry:
• Unit Cell Chemistry ...
_________________________________
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections







Final Exams and Video Playlists: www.video-tutor.net/
Full-Length Videos & Worksheets: www.patreon.com/MathScienceTutor/collections
Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
how does it feel to be a legendary chemistry teacher
he's a jack of all trade actually!
He just pure legendary he taught Virgin Mary how to have a baby and remain a virgin
Feels damn gooood !! Thanks for asking
@@Sir_Emo i didnt know wether to like ur coment or go to heaven
I learned Calculus from him before I learned chemistry, this man is truly the best teacher on TH-cam
This man has so much power in my life. He could totally tell me the wrong this and I would believe it without question
hi just letting you know that your videos are the only reason I'm passing gen chem 1. you're the best.
Thank you so much for your kind comment on our videos. I'm hoping you are now a Chem 1 master.
@@musick4288 who da fook is this guy
I was totally confused after attending my teacher's lecture on Resonance Structures. I couldn't understand a single thing, I got so irritated and came here and within 10 mins 30 seconds I have now mastered the concept! (okay, yeah, atleast to some extent) He is so calm, composed and clear headed that his attitude reflects into yours too, even through the virtual medium! I'm not the kind that usually posts comments but this video definitely deserves applause... Way to go sir @The Organic Chemistry Tutor
I don't understand it, maybe I'm missing something 🤔
It's been 8 years since I took high school chemistry. I'm a junior in college now. My Chem professor said, "If you don't remember how to do high school chem you're going to struggle in this class. And I did. Then I remembered you're channel existed and have had no problems learning the material. I'm starting to think that she was just trying to make excuses for not actually teaching the material.
What my prof taught in half a lecture, you taught me in 3 minutes. Literally at the 3min mark the lightbulb went off in my head, thank you kind sir.🙏🏻
I have found a more developed way to get the number of lone pairs, here’s how it works - First count the number of valence electrons, just like he does but if you have have Hydrogen in the molecule, count its valence electrons as ‘7’. Now that you have the total valence electrons, divide it by 8. Now you will have a quotient and a remainder, the quotient tells you about the number of sigma bonds in the molecule and then divide the remainder by 2 and it will give you the number of lone pairs on the molecule(Just try it once, although it only works on single central atom species). You can even use this to get the HYBRIDIZATION of a molecule. For example take NO2- , here VE(N) = 5, VE(O)=6 and count the electron as 1, so total we get 5 + 2 x 6 + 1 = 18, now divide by 8 we get 2 as quotient and 2 as remainder, that means, there are two sigma bonds and 2/2=1 lone pair on the central atom, And the fun part is if you add 2(sigma bonds) and 1(lone pair) you get three which states that it is a sp2 hybridized molecule. ( 2= sp , 3=sp2, 4=sp3 and so on) good tryy ittt. Thank you for reading and do try it with a hydrogen species as well, just remember to count its valence as 7. And channel owner sir please pin it as well so others can be helped as well😷👍
This guy has taught me everything I know and I don't even know what he looks like lollll. Man needs a university named after him.
My goal is to finish watching (learning from) every video you've uploaded (regarding chemistry) before completing my senior secondary edu 🐸
Hi, i am 13 yrs old and you helped me for my researches about resonanse structures, thank you
You are the reason I am passing General Chemistry. Thank you so much
Bro no bullshit, u deserve a noble peace prize for helping thousands of chem students pass they’re exams
It is really sister
incredibly helpful ......your videos are the only reason to make me enable to pass my course of organic chemistry
i honestly needed this for my midterm, thank you!!!!
Thank you so much! You make me love chemistry even more!
The way a TH-camr explains a difficult topic is just like raising a new born baby by her parents.
Btw thanks a lot
Professor Organic Chemistry Tutor, thank you for a basic Introduction into Resonance Structures in AP/General Chemistry. Drawing the resonance hybrid is not a difficult process, however moving the electrons / lone pairs around can be problematic. In drawing resonance forms, you can only move the electrons. This is an error free video/lecture on TH-cam TV with the Organic Chemistry Tutor.
I’m finally taking o chem!! Even though I’ve been watching my whole college career for stats, algebra, and other math courses! Thank you
Our prof is redirecting us to you. We do not use platforms for online lectures due to poor internet connection. Should we give you her salary to you instead?
😂
This guy has taught more people than some professors lol
plus more easier to understand than most professors lol.
some? more like most lmao
love u bro. always helping out others
Bro thank you for the kind comment but we, the Organic Chemistry Tutor, don't love you.
@@musick4288 impostor😂
@@michelletorres4926 Nah, I'm a crewmate. hihihi
..... You're the next Albert Einstein 📉🧠🖤😅
You the best bro
Genuinely taught me more than my teachers at school.. now i finally understand why we gotta find lone pairs and stuff
how simple you make chemistry !!!
when i practice exam i always look at ur videos when im facing difficult questions and its helping me alot
so thanks
He really simplified it!
U deserves 2.3 M subscribers❤️
He's got more now. 2.8M
@@anvayaiyer5614 ❤️🤩
4.55 :)
thank you so match, had a hard time to understand that until now
You are so match welcome!
Like 👍 if he is better than your highly paid chemistry lecturer.
I was at first trying to relocate certain atoms and ions to other parts of the structure, but realized that that would turn the molecule into another molecule or a molecule that doesn't exist. So better do it the more labor intense way and draw all the resonance forms:)
my ochem professor makes us not put lone pairs on the resonance hybrid. maybe just a preference thing? patron here. best science videos ever!
Always great videos. I've watched you for algebra too!
OMG thank you this was such a huge help
I follow you forever. From a ChemE student.
Can we all agree that traditional chemistry professors basically suck at what they do
like wtf. This guy makes it so simple.
Please don't use dark-blue colored pens on a black background. It's hard to distinguish between the two. Great video.
fuck u bitch. why dont u make the video then
@@PHILLYMEDIC69 he asked him politely and shared his opinion. there's no need to be harsh on him.
lol he's so mad
@@fromir6619 JEBAITED
@@PHILLYMEDIC69 LOL GET THE STRAP
You are making difference in peoples life thank you teacher
Thank you so much for your help,your videos really are helpful
You speak to my polymath heart!
my chem exam is in 2 hours and im cramming 😵💫
u are the only god i praise
Thanku sir i have learned draw resonance structure finally
thank you for telling me that lone pair formula only works if hydrogen isnt present... legit been asking why this formula he showed isnt working on a number of problems I have been working on.
Thanks! Incredibly helpful!
You help me alot man keep it up!!! ❤️❤️
Short and simple. Thank you.
Great help. Thank you.
Believe me or not but your voice is so beautiful 🤩
It helps me a lot😊😊
Let's watch his ads to help him back!
Y????
Mad one !
@@karrivijayakumar1466 you can make money off of TH-cam ads
BEST CHEM TEACHER 🙏🙏🙏🙏
Good luck with chapter 8 😂❤️
amazing video
Thanks for the video sir💖
Excellent service
I really understood this thank you very much
Thank you!!!
You make it a lot easier to understand :)
thank you very much for this video
easy and good explanation
Amazing!😭🙏
great teacher... thank you soooo much
How do you know where to place the charges on the structure? I know you said oxygen always has a - charge if it has 1 bond and 3 lone pairs, but does that rule apply to other elements? thanks!
Wow! Thanks for this one
Learned in only 8 minutes than the prof. taught in 1 hour
Will this also work for ap chem?
finally i can really understand itttt
many thx
You my best man
You are the besttttt!!!
Thank you so much; I benefit from your video by building highly effective learning.
How to know if a lewis structure really needs resonance?
Thanks for your offer
Thank you!!!
In really appreciated this vedio...tnx very much
Please make a vedio of showing how to draw resonance structure for NO2+( nitronium ion) I'm so confused With it.
THANK YOU THANK YOU THANK YOU
At 2:03, is there a reason for why Oxygen has a (-) when it has 3 lone pairs?
I think you'll find the answer in a video about "formal charges".
thank you so much
very useful!!
DOUBT:
HOW CAN WE ADD 3 LONE PAIRS TO AN OXYGEN ATOM JUST BECAUSE IT HAS ONLY ONE BOND WITH THE CENTRAL ATOM? (an oxygen atom only has 6 valence electrons, and after using an electron to bond it would get 7 electrons in which 4 are the electrons present in the 2 lone pairs and the other 2 electrons are the ones present in the covalent bond, where does it get the EXTRA ELECTRON to get 3 lone pairs?)
Thanks you so much for everything but I wanna you to do a video of how to identify the number of canonical structures
Why don't you put the negative charge of NO2- on O but in brackets... ?
Thank you Sir
Hi I have a question. How would you know that building a resonance structure would stop at a certain point? Foe example, in your 1st ex the Co3 2- you got 3, but in No2 you only got 2?
If the same resonance structure with which we started repeats then we can come to a conclusion that there are no more resonance structures possible for the given molecule
The comment above me is correct. This is because Co3 2- has THREE oxygen atoms, so the double bond will move between three oxygens. But No2 has TWO oxygen atoms, so the double bond will move between two oxygens only. Hence, Co3 2- has three resonance structures, and No2 has two.
Hope this helped. :)
@@anabanana8518 thanks guys. I'm now at 2nd year college. I think we're having a biochemistry course next sem after this pandemic.
ACMD so cool!
@@anabanana8518 Oh my gosh, Ana that's cool!
Wow,,your awesome,,thanks❤
It's amazing
very helpful
When you break the first N-O double bond, are you not supposed to evenly distribute the two electrons from that bond between N and O, in which case the oxygen ends up having 2 lone pairs and one unpaired electron instead of the 3 lone pairs on the diagram??
❤❤❤you are the best
At 5:58 he says to draw the nitrate structure but writes down the nitrite NO2- ion 😅
Love your videos.. that’s nitrite not nitrate..
Is it a must to add the lone pairs in the resonance hybrid?
Thank you sir🙏🙏🙏🙏
Thank you
Sir I have a doubt , is resonating always have coordinate covalent bond?
what is the difference between resonance structure and lewis structure? I really need to know in order to survive on my midterm test🙏🏻
can someone explain like what is the overall goal in resonance structures like how do we know which e- is going to which atom
bless ur soul
1:59 is there any way to know that? Or is it memorization
Gud understnding video