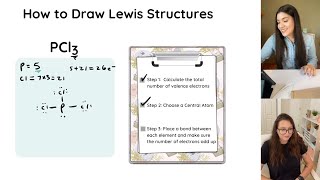
Lewis Structures and Formal Charges Practice Problems | Study Chemistry With Us
ฝัง
- เผยแพร่เมื่อ 26 ก.ย. 2024
- Practice drawing these lewis structures and don't worry we will go over all the answers step by step. This video will explain how to find the formal charges of each element in a structure, what formal charges are best, and why formal charges can be used to draw the best lewis structure.
📝 DOWNLOAD THE STUDY PLAN
melissa.help/s...
📗 FREE CHEMISTRY SURVIVAL GUIDE
melissa.help/f...
📘 FREE ORGANIC CHEMISTRY SURVIVAL GUIDE
melissa.help/f...
💯 HERE'S HOW TO PASS ORGANIC CHEMISTRY 🎉
chemmunity.inf...
👉 MORE CHEMISTRY RESOURCES I CREATED 👈
melissamaribel...
🎓 CHECKOUT MY COMPLETE CHEMISTRY GUIDES:
📕 Thermochemistry Guide
melissa.help/t...
📗 Acids and Bases Guide
melissa.help/a...
📘 Naming Compounds and Acids Guide
melissa.help/n...
📙 Dimensional Analysis, Significant Figures, and Density Guide
melissa.help/s...
📕 Gas Laws Guide
melissa.help/g...
📗 Stoichiometry Guide
melissa.help/s...
📘 Redox Reactions Guide
melissa.help/r...
📙 Molarity Guide
melissa.help/m...
📕 Limiting Reactants Guide
melissa.help/l...
📗 Lewis Structures Guide
melissa.help/l...
📘 Kinetics Guide
melissa.help/k...
📙 Titrations Guide
melissa.help/t...
📕 Matter, Atomic Structure, Empirical and Molecular Formulas Guide
melissa.help/m...
🙌 This was my go-to homework help when I was in school. Chegg Study is one of my favorites.
melissa.help/c...
📚 I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Thankfully you do, with Chegg Textbook Rentals.
melissa.help/c...
💁♀️ HI I'M MELISSA MARIBEL
I help students pass Chemistry and Organic Chemistry. I used to struggle with this subject, so when I finally graduated with a bachelor's degree in Chemistry, I became a tutor so that you wouldn't have to struggle like I did. I know that with the right help, YOU CAN LEARN ANYTHING!
DISCLAIMER: Some links in the description are affiliate links, which means that if you buy from those links, I’ll receive a small commission. This helps support the channel and allows me to continue making videos like this. Thanks for the support!








you are my savior!! My chem slides didnt even mention this part and im paying nearly 1k for this class. Thank you for helping other students especially during this pandemic where having a one on one with a professor is difficult nowadays.
Jesus Christ is our Saviour
Need to find a better class I am sorry yours is bad.
Ikr!!!!! I have a short test on these tomoro
This was SUCH a big help!! Thank you so much!!
I'm sad that you don't have more views on this! Everyone needs to watch this video!
You're an amazing teacher, thank you very much for helping us all! :)
Thank goodness for you! Helped me SO SO much! You are definitely one of the best tutors on TH-cam!!
Omg I can understand this more easily than my teacher's explanation. Youre a life saver! Haha
Yea
Me too✨✨
Thank you so much for doing these! It is like getting one-on-one tutoring, and your student asking questions and you including your answers helps so much more than just someone talking at me the whole time. It is so nice. This is a lifesaver find on the internet for me!! xD
thank you!
I can't believe this is free to watch, thank you so much!!!
It always takes me so much time to get myself to sit down and actually study for chemistry and you always help me in completing my studies. Thank you so much!!
A very perfect way of showing the student solving the problem and as the student moves on, giving instructions. My students learned a lot from that. Not many have a youtube whereby the student is active solving a formula. Very very very good. compliment.
I literally have an exam today and this just saved me. Thank you!!
You are AWESOME! Having a student sitting in was super helpful, too! College life saver
you've made this more clear. Thank you Melissa
THIS MAKES SO MUCH SENSE NOW THANK YOU
You saved my life!!! Thank you!
I'm so greatful, you helped me with my chemistry exam next week...
Melissa, you put in a lot of sincere efforts in preparation and lucid teaching.. Kudos..
These are brilliant thank you so much, you have no idea how much youve helped. and you explain everything so well
I'm sure with this, I'm not failing my tests. Thank you so much... I can't believe I fully understand what has been giving me headache, I'm so teary right now
Melissa, I grew up in a cult where we weren't allowed to learn Science at all. I recently started college and was terrified of how I'd do. Over the summer I watched all of your videos to prepare and I just took my first Chemistry test ever and got a 95%. Thank you!!!!
wow
This kind of video set-up is super helpful! Thank you Melissa
Thank u very much, that helps me alot, this is the first time i understand why there are more than one Lewis structure for polyatomic ions.
The notes are so pretty I can’t stop looking and by extent I’m actually learning!
This was a HUGE help for my incoming exam!
Thank you so much!
Love from Italy
Thank you very much, no more questions about this again.
Never would've thought watching this kind of tutoring videos would help me understand something better!! that's it, gonna go for "with us" rather than "with me" from now on
GOD BLESS YOU OMG I'm taking summer gen chem at my community college rn and its been so stressful the professor barely has time to cover everything so she had to rush through this unit and I was honestly trying not to cry on the way home 😭 watching this video definitely cleared up a few things for me and I'm feeling a lot more confident, my exam on this is in 2 days
i think i need a little more practice and ill be where I need to be to get that A !!!
Melissa is the best teacher I have ever met. Thank you so much for making the effort to change our lives!!
You're the best!!! You literally NEVER fail me :)!
Everything made sense ma'am you are amazing ❤️❤️❤️❤️
THIS VIDEO WAS A LIFE SAVER OH MY GOSH TY
Video was so helpful! Thank you!!!!!!
thank you so much I went back to the uni after 3 years dropping off and was forgetting all the chemistry I learned back in high school, this helped me a lot. : )
Thanks, I have better understanding for formal charges after watching your video.
You are a great teacher 🤗
Have really understood wooow so happy thanks mrs
Thank you... I have just finished my quiz successfully. Since now, I am your new subscriber.
Hello Melissa, this video really helped out. Thank you!
Extremely useful video for my students. Make more on other topics also !
I am from phillipines and an I.E student from pup, thank you for having a detailed discussion about this topic especially now a days, we're just finding our own way to learn by ourselves and tbh, it is really hard.
Really helpful 🙏🏼♥️
I really like these kind of problem solving videos
Using this adding formula instead of the fraction formula is so much easier. Thank you.
Thank you! have my finals tomorrow.
I got all of it just right. Thanks!
This is so helpful, TQSM!
This video helped me SO much!!! Thank you, thank you, thank you!
Thank you so much. I just found this one when I am very stuck with Lewis structure
Thank you soooooooo much!!!!!
Their is a trick I got from this whole lesson
That is ,that when their is just a single minus sign that means I will have just one single bond in the structure and that also implies to the sign of minus 2
Thanks for this great video
Really you are so good your explanation is very very good and when you was teach I just kept looking at you honestly you are so good teacher and my guide
i do not know how you don’t have more views, you are fantastic!
Thanks a lot for the video .This was a great help for my studies.
you are amazing thank you for saving me from my college complicated assignments.
this helped a lot...thank you
Hi, thank you so much I get it more! But one question, I thought that the formal charge formula looks like: FC= #valance electrons -(#non-bonding + 1/2 bonding electrons) because that’s what I’ve been learning. So is the 1/2 not necessary?
Thank you so much though !!!
1/2×5 is the same this as 1/2×5/1
So the 1 times the bonding electrons
احسن من شرح دكتورنا
I just found this video, THANKS! I wish i had a teacher like you, you are awesome!
U are truly a savior 💯💯 I love u❤️🥰
A great tutorial....students usually have problems with octet and expanded octet and fc....but the explanation was great...well done and thank you
Some great stuff! very easy to follow along, thank you very much!
I have a chem quiz tomorrow on Lewis Dot Structure and I was super worried about it, but now I'm not worried at all. Also, I never comment on youtube videos but you are such a great teacher that I had to say thank you!
Melissa the wise giver❣️❣️🕊️🕊️📝
This shit is the best, rarely you see vids that tutor, keep going you goats, also nice editing, love how much work you put in!
It would nice to have an OCHEM basis video.
It's very helpful 😍 it would be great if you could give more practice problems, thank you so much!!
You always help me
What do you do if, in order to make the central atom have a formal charge of zero, you can't meet the octet rule? Which is more important if you can't do both? For example- CCl2. To make carbon have a FC of zero, carbon will be left with only 6 valence electrons and carbon is not an exception to the octet rule.
Thank you so much✨✨
I understand much more💕💕
At 7.01, how come the oxygen atoms have 6 lone pairs of electrons attached, as well as the one bonding with Sulphur, which adds up to 7, if it only has 6 valence electrons?
I have the same question
Thank you for your content! It has been very helpful!
I actually am confused about the very last problem. Why wouldn't nitrogen and oxygen have a double bond and then fill in the lone pair for oxygen? When do you use double bonds versus lone pairs?
Thank you so much
Thank you melissa it was really helpful!!❤ i could nt understand one part at 24:48 why one of the Fluorine atoms have only two lone pairs i understand why the other Fs have 3 lone pairs but i don t get why this F has 2 lone pairs
You are THE BEST! Thank you!
Your formal charge equation I prefer over the book! Thank you so much I understood it better practicing with you.
Thank u sooo much , it helped me a lot !
HIIII Melissa, I just wanted to ask if it matters where we put the hydrogen for the oxygen in the last problem?
so freaking helpful, thank you!!!💛
Can you make a video about functional groups? PLEASE AND THANK YOU!!!
Thank you for this video. However, I just had a quick question I thought that the central atom always has to have a charge of zero. Because for No3- the charge on the nitrogen is 1 and thats the right structure. So how would you know if the central atom is supposed to be zero or have a charge on it? Thank you in advance
Can anyone explain when she does [SO4]-2, why sulfer does not form only 2 bonds? i thought since it was in group 16, it would only want to form 2 bonds, not 4. Thank you!
why is it more favourable to have a neutral formal charge on the central atom? do you have a video about this?
That's amazing thank you so much
I cannot thank you enough!!!!😭😭😭😭😭
gracias!!
Thanks
Thanks from India mam
Thank you so much mam
Melissa pls explain why sulphur is having 6 bonds because it only makes a bond
On the NH2OH problem, could we have triple bonded oxygen and nitrogen with oxygen having one lone pair?
no, because in this Nitrogen will have 10 electrons around it and it cannot expand the octet.
học đến cuối năm rồi giờ mới hiểu được bài này
What do we do if the formula has an element like Ti in it? Like TiCl4
Ma'am how can we draw lewis dot structure for Br3O8 ma'am? can u pls explain it?
thanku mam it helps a lot
any have an idea why the last question at 27:50 can't have a double bond between the Nitrogen and the Oxygen with the Oxygen having 4 electrons on it still
so helpful!
why does cl go next to C and not next to H??? I dont know if my question makes sense lol, but like I wanted to draw the hydrogens around the carbon then put clorine next to one of the H but here you say to still have the hydrogens around C but leave a space open to attach that Cl
What is the device you use to show this awesome exercise online?
I applied the formal charge rule and in most cases, it worked. However, it is not working for NO3-. I thought there should be 5 bonded electrons attach to N but the correct one is actually 4 bonded electrons attach to N.
Love u Melissa ❤️
thank you!
Please upload a video on acids and bases,my favorite teacher
it was good..