SN1 SN2 E1 E2 Reaction Mechanism - Test Review
ฝัง
- เผยแพร่เมื่อ 15 เม.ย. 2021
- This organic chemistry video tutorial provides plenty of multiple choice practice problems on SN1, SN2, E1, and E2 reaction mechanisms.
Access The Full 4 Hour Video: / mathsciencetutor
Direct Link to The Full Video:
bit.ly/3Bt4ghw
PDF Worksheet - 77 Questions:
bit.ly/3J1exrP
Organic Chemistry PDF Worksheets:
www.video-tutor.net/orgo-chem...
_______________________________
Join The TH-cam Membership Program:
bit.ly/46xaQTR
Full 4 Hour Video on TH-cam:
• SN1 SN2 E1 & E2 Reacti...
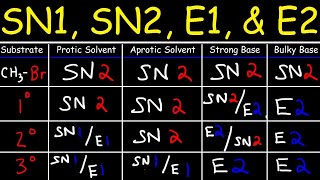

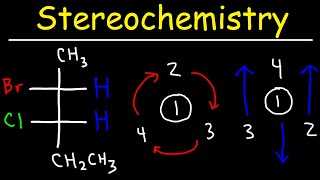
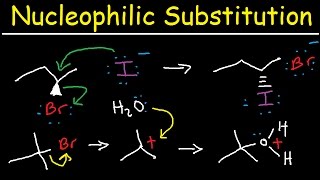





Organic Chemistry PDF Worksheets: www.video-tutor.net/orgo-chem.html
Full-Length Exams & PDF Worksheets: www.patreon.com/MathScienceTutor/collections
Access The Full 4 Hour Video: bit.ly/3Bt4ghw
PDF Worksheet - 77 Questions: bit.ly/3J1exrP
This came out just in time, got a test on this stuff tomorrow
I badly need this lesson. Thank you so much for uploading before my midterms!
i'm very curious as to how you know so much about everything in other subjects
He is a professor and probably majored in chemistry, which requires a lot of math, chem, and bio knowledge
You get prepared for the subject, work hard on it, practice and if you have some talent on the subjects BAM! You've got an informative video.
@@tsugumilana7581 also he might have notes which most college professors have and probably keeps updating it. That’s what I do as a tutor. It takes practice
I'm a chemistry major. In addition to 50+ hours of chem, math thru calc III is required, and calc based physics
it's all based on each other, it just works
i appreciate you uploading and taking the time in doing this lecture. this really helped clear things up.
This is so helpful! I love the way you explain this using practice questions
😺
Just what i needed thank you!
Nice problem set containing a wide range of different types of questions related to this ever confusing topic.
Thank you so much.
I'll have exam on this on Tuesday
What a great TH-cam channel, amazing!
I like your videos they are very helpful. I have been out of the loop so to speak, when it comes to organic chemistry for at least 20 years. I just wanted to point out that around seven minutes into the video on your multiple-choice format the isopropyl benzene that has the bromine leave to form a carbocation you have it as choice C. The formula appears to be one carbon short. I believe it should be as follows .... C7H5 (CH3)2. This is because I count and total of nine carbons, not eight. Anyway like I said earlier your videos have been very helpful thanks again.
I remember watching this a year ago for my OChemII class. So helpful for this topic especially where there are so many contradicting ideas.
you seriously saved my Ochem score!!!!
have a test in exactly one hour this is perfect
thank you for getting me through college
when this is only ONE part of your test :(:(
just on time!
Iam a big fan of your explanation I understand your method of your solving with ease which is a rare gift you have .tqq so much ❤❤❤😊😊😊😊
Love from India sir.🇮🇳🇮🇳
Oh my god your a lifesaver :’)
so very useful
So helpful. Thank you so much!! Great review, with step by step explanation. Orgo Test #6 tomorrow. Then the final. Whew! So over it!!
Thank you man ❤
So understandable sir
Please sir which text book u are using
Hello! In 21:23 you said that iodide ion is a strong nucleophile. But how can it be strong if it is in an aprotic solvent, where among F, Cl and Br ions its nucleophilicity has the lowest value? Or is it possible that despite being least nucleophilic among F, Cl and Br, it still has a high value of nucleophilicity?
i’m not an expert but my professor always says that if it’s charged, it’s a strong nucleophile. since it’s an iodide ion, it has a negative charge. the aprotic solvent will actually help it, because a protic solvent would surround the nucleophile and hinder it. that’s why SN2/E2 reactions favor aprotic solvents
Despite it being the weakest nucleophile of the halogens in an aprotic solvent it still is a relatively strong nucleophile
39:45 The methanol molecule extracts the proton? Not the chloride ion?
Hey, why didn't you do methyl shift for #3?
ARE YOU SOME KIND OF MESSIAH !!!!!!
54:59 Isn't it HSO4- that extracts the proton, hence the acid catalysis?
At 21:54 why is the I- considered a strong nucleophile when the reaction is occurring in an aprotic solvent?
hmm good catch
I was thinking the same thing since on 14:33 he said I- was not the strongest nucleophile (for aprotic solvents)🤔
15 seconds in and I've been called out.
I clicked on the video you recommended but it says for members only so how do I become a member?
There is a join option next to subscribe.
for question 10 at minute 39:00, he says that we can't use sodium methoxide to make the product. im confused by this, how does he know sodium methoxide will give us an elimination product, is it because the NaOCH3 is protic? or is that wrong help plz
I think it is because OCH3 on its own is a strong base so it will be more likely to take a hydrogen from the substrate and do an Elimination than act as a nucleophile and do a Substitution
واو على وقتك
Hey, I love your videos, they are really helpful! I have a question though, on number 9, how come water would work better than option A for a SN1 reaction? I thought SN1 favored tertiary carbons.
Compounds that are tertiary will undergo a SN1 reaction more readily. In this case, we were looking for what solvent would allow for that reaction to undergo which relies on polarity of the solvent which water is the most polar in this case. Basically tertiary compound is the one going through the SN1 reaction while water is the one causing it.
Shouldn't the reaction in question 11 be E1 mechanism because it has polar protic solvent as medium ? I just can't figure out why it is E2 ? At 40:35
That's more than likely a mistake there, if he had used some other polar protic solvent like water then it would've been correct, because water is so small to solvate tert-butoxide anion and so it would've still been an E2 reaction, even though the solvent is polar protic always look at the size of the anion of the base also to decide whether it's an e1 or e2 reaction, so yes u r correct it should've been an E1 reaction because tert-butanol is used as solvent which has the capacity to solvate big anions
8:32
I thought you said strong nucleophiles like Iodine favors SN1 reactions and Fluorine is strongest for SN2 reactions. How then is iodine a strong nucleophile for an SN2 reaction here in question number 6? I am confused.
I believe that in any Polar solvent that once the Bromine leaves the Iodine can attach easily because it is able to bully it’s way around the positive end of the acetone trying to inhibit Iodine’s movement because of it being a larger molecule. Fluorine is the strongest but smallest in size so it would have the most difficult time trying to go around acetone molecules. However, in Non polar solvents, then electro negativity wins out and then Iodine couldn’t replace Bromine. However I don’t understand the Bromine being pushed out by an iodine in an SN2 reaction either so I agree with you on that. It seems Bromine would be the one replacing Iodine.
❤️
Hi
is #12 also an E2 rxn?
58:46 Anyone that thinks the answer is C?
xoxo
Firstt
First view bro ❤️
Can I see u bruh
Stop reducing videos duration...