Nucleophilic Substitution Reactions - SN1 and SN2 Mechanism, Organic Chemistry
ฝัง
- เผยแพร่เมื่อ 26 พ.ย. 2024
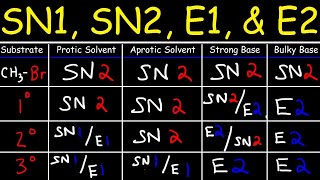
- This organic chemistry video tutorial explains how nucleophilic substitution reactions work. It focuses on the SN1 and Sn2 reaction mechanism and it provides plenty of examples and practice problems. The Sn2 reaction is a second order nucleophilic substitution reaction where the rate is dependent on the concentration of the substrate / alkyl halide and the nucleophile. SN2 reactions occur with inversion of configuration and work well with methyl and primary substrates. It's a concerted reaction mechanism that occurs in a single step. The rate law for the SN1 reaction is given as well. SN1 reactions proceed via a carbocation intermediate and carbocation rearrangements such as the hydride shift and the methyl shift are possible. SN1 reactions work well with tertiary alkyl halide substrates due to carbocation stability. Carbocations are stabilized by means of hyperconjugation and the inductive effect. SN1 reactions will produce an unequal racemic mixture. The stereochemistry of both reaction mechanisms are discussed in detail. SN1 reactions work well with polar protic solvents but SN2 reactions work better in polar aprotic solvents. Solvolysis reactions are sn1 reactions where the nucleophile is the same as the solvent.
Stereochemistry R/S Configuration: • Stereochemistry - R S ...
Optical Activity & Specific Rotation:
• Optical Activity - Spe...
SN1, SN2, E1, E2 Reaction Mechanisms:
• SN2 SN1 E1 E2 Reaction...
SN2 Reaction Mechanisms:
• SN2 Reaction Mechanisms
SN2 - Test Question:
• SN2 Reaction Mechanism...
_______________________________
SN1 Reaction Mechanisms:
• SN1 Reaction Mechanism
Carbocation Stability - Hyperconjugation:
• Carbocation Stability ...
Carbanion Stability:
• Carbanion Stability
Protic Vs Aprotic Solvents:
• Polar Protic Solvents ...
E1 Ring Expansion:
• E1 Reaction Mechanism ...
E2 - Test Question:
• Zaitsev vs Hoffman's P...
________________________________
E2 Stereochemistry - Newman Projections:
• E2 Stereochemistry Wit...
SN1, SN2, E1, E2 - Practice Test:
• SN1 SN2 E1 E2 Reaction...
Organic Chemistry PDF Worksheets:
www.video-tuto...
Organic Chemistry 1 Exam 2 Playlist:
bit.ly/3PKEApB
Full-Length Videos and Worksheets:
/ collections








Organic Chemistry PDF Worksheets: www.video-tutor.net/orgo-chem.html
Full-Length Exams and Worksheets: www.patreon.com/MathScienceTutor/collections
SN1 SN2 E1 E2 Reactions Test Review: bit.ly/3Bt4ghw
Organic Chemistry Final Exam Review: bit.ly/2WCJ8GP
thank you goat
"let's not worry about stereochemistry in this example"
I think that is the sexiest thing a chemistry teacher could ever say
Orgo 1 final exam tomorrow, and I know I'm not alone. For those of you cramming like I am, I wish you the best of luck. One final push, comrades. Let's do this.
Its friking hard
@@gigachad5029 its awesome its kinda like math word problem its like solving a puzzle just look at it as if its a puzzle and make up million possibilities in your head then analyze them closely then take the one which makes the most sense play the video and oh boy when u are right that feeling u get is so lit
@@akashsunil7464 still annoying thi
@Orion Jaiden Bot says what?
i hate organic cant wait ti get this over with
I never skip the ads because that's the only way I can pay you back for literally making orgo MAKE SENSE! THANK YOU!!!
dude, i just want to say your channels are literally the reason im surviving chemistry right now. Thank you! I watch your channel basically everyday.
na i am very smart
@@Jdkrjj ?
You teach better in 15 minutes then my "professors" who leave me so confused after 2 hours.
Sure. Same for me. Professor said: you will learn it later on lessons. For me, It feels like pre-view and "waste" of time.
@@kevinhan2581dude the amount of times my proff said “keep this in mind it doesn’t make sense now, but it’ll make sense later”
And half the time never comes around to what he meant. randomly he says “see remember what I told you this would make sense in a bit which is now” and elaborates nothing on the topic leaving it a mystery beginning to end.
Recently it was nucleophiles before it’s chapter and bro swear it was some mind blowing thing we wouldn’t get yet. 2 weeks later it’s just e rich species. Niceee set upp.
I hope this man gets everything he wants in life
💯 always saving lives!
@1:46 you say "the SN1 reaction occurs in a single step" For anyone else thatt might be reading this, it's supposed to say "the SN2 reaction" not "SN1"
yes
Yes
I was wondering about that. Thank you!
Yes, I just caught that as well!
I wish I would've read this comment 30 mins ago!!! I had to pop out my textbook cause I was so confused!!!! Thank you! two years later lol
yea... im fucked
brandon mora I’m with you brother
i have my chem test in 5 hours
AAARHHH how’d it go?
@@aaarhhh4341 12 hours remain for me
lmfaooooo
I have to criticize a bit. Sn2 has 2 in its name not because it is second order overall but because it is a bimolecular nucleophilic substitution, emphasis on bi. Sn2 can occur as a pseudo-first order reaction in case of solvolysis(when the solvent is the nucleophile and is in excess)! But otherwise, thank you for this video.
bimolecular literally means it is a second order reaction
@@okayhaffy no rate = k [a]^2 you would say this is still second order ,I thought. (I could be wrong)
My Doctorate lecturer, I just take this opportunity to express my sincere gratitude for enabling me to pass my exams and cats. Actually, you are a hero to be remembered. I revised all the problems and guess what. Exam was just a celebration!!. Long live my master.
the best organic chemistry tutor ever
Biology is my subject, i was never really good at chem, but watching your videos clarifies alot.
This channel is doing the Lord's work. Thank you.
You guys have no idea how I've been battling to get the Sn1 and Sn2 reactions. Thanks
organic chemistry tutor i would trust you with my child
seriously..just 18 minutes and concept is so damn clearrrr!!!
Your videos make me understand everything SO much better than the classes I pay for do. Thank you so much
The amount of times youve saved my ass is out of this world. Youve basically been teaching me my whole college career. Thankyou ❣
Im here starting college yes he is saving us FR
What year are you in? Personally in my school (Belgium) I’m learning this during my final year of highschool so I’m wondering
@@nauwwww bro same but not from Belgium
You save my life! I have to give in a test on this in like 2 days.
Am going to write my exam today and I know this will help because I have been watching it since, thank you
I might actually pass my exam so thanks, I was dead without this.
Ayo you’re such a legend. I didn’t understand shit in school and now after this video i understood most of it.
Giving u my first born child
Thank you so much 😊,all the organic class’ students in class admire your teaching way.
this is very useful. Thank you for the wonderful explanations as always!
We Need you all the time for every single topic ❤
Wow! I love your channel! You helped me a lot with AP and now you're helping me out with even IIT!
Thanks!!
You're saving me in this first semester of Ochem
Sir you so amazing my teacher makes it more complicated n I came to a conclusion that chemistry is hard but u took me to another level
Understood more in five minutes here than I did from 2 weeks (about 4-5 hours) of lectures from my ochem 1 professor. .
Every winter this man re-emerges to save us from finals
I hope this isn't too forward but... I love you.
tbh i learn more from your videos than from my actual professor
preci preci i now have a girlfriend and a 102 in organic chemistry thanks to ur videos. also own a lamborghini and live in a gated community in the hills thanks to your hard work
I hope you know how important your videos to us are. Thanks
Good work, but it was very confusing when you switch from sn1 to Sn2 without saying anything so i cant tell which is which
First look at the Alpha C. Is is a primary, secondary, or tertiary C? If it is Primary, it will always be Sn2 (minus a couple instances), if it is secondary you need to look at what you are reacting with and the protic/aprotic conditions along with the leaving group, if it is tertiary is always favors Sn1.
It’s implied when we discuss sn1/sn2. The biggest thing to remember (what I learned) is that sn1 is usually a 2 step process while sn2 are 1 step:) hope it helps. There’s basic rules to help you immediately see the rxn that will happen when u see the reagent with starting material. So primary, sec, and tertiary substrates helps me know whether the rxn will proceed in either sn1 or sn2. Check the Carbon:)
@@yasssgawwwd5643 bruh that message Is2 years ago, and he just made some mistakes in the video tbh said sn1 when he meant sn2
@kuewayneennis9521 Yes! I was also confused because at 1:47 he says "The SN1 reaction." while he meant SN2! I hope there aren't more confusions?
Also how ironic rn, your comment is 2 yo for me 😂😂
In your first example, isn't iodine a better leaving group, and thus a weaker base, than bromine? So the reaction will not proceed, or will at least favor the reactants if the reactions is reversible
I love this channel so much
I love your videos, they're informative and kinda asmr lol thank you so much.
Its me from Nepal
Your videos are extraordinary sir 🥰
Hello! This was awesome, thanks alot for your tutoring!! It helps alot😁
magnificent, all 360p's of it
thanks for your videos dude, very helpful!
correct me if I'm wrong, but I'm pretty sure that the second example can only work with E2. SN1 does not work under strong basic conditions.
Substitution reactions prefer formation of the more stable base. Reacting iodine with an alkyl-bromide would produce no reaction. Please clarify this
Why does the intermediate molecule at 14:21 let go of the hydrogen instead of the methyl group? What makes it more favorable? Why would the methanol want the hydrogen? Wouldn't it have to dissociate for the OH- to grab the H+ and become H2O, leaving a methyl ion CH3+, which is kinda unfavorable?
the fact that i can understand him and not my professor!!!!
hi not sure if you can see this comment since this is a pretty old video,, but im open to anyone who can answer my question: for 17:43 product, is it not going to be a racemic mixture just like the previous example since H2O is a neutral nucleophile?
There are a few strange things:
1. I is a better LG than Br so it shouldn’t react
2. Methanol is acidic and would not accept protons
this video helped me out a lot
Overall, this video is very helpful. However, I'd like to point out that at many points in the video, some terms are not very clearly pronounced.
1:46 don't you mean Sn2?
Yes SN2 is Concerted single step, while SN1 is multi step.
That's what I was thinking too
Does Carbonation formation occur in SN1?
I need to know this for a test in less than 1.5 hour. Thanks.
My guy, you are a B L E S S I N G
you are the best!!!!!
hmm why these guys are explaining things to us on youtube?!! this should be my professor
Thank u so much for this video
i tought I- was a better abandonat group than Br- . but in the video you use Br- as the abandonat group. i am kinda cofused. can somebody explain?
Is this for the AS level?
does SN2 and SN1 work for alkane, alkene and alkynes or only for alkyl halides?
how do you know if it attacks from behind or in front
Very good effort👍
Thank you so much sir !
on the last problem, how do you which is r and s? there's no methyl on a dashed or wedge bond.
Very helpful
What is the link to video that has 75 practice problems?
For the last molecule, would it actually have 4 steroisomers? The methyl-substituted carbon is an asymmetric center, and I assume the methyl shift could add to either side.
Not me checking how to do this 5 minutes before the exam 🎉
My exam on this is today 😂😂
at 4:05 you were drawing 2-bromo-2-methylpropane but as you can see that you made mistake for that methyl (to the left of this molecule.) C and H suppose to be switched or else I will think that the carbon in the center of the molecule is connected to the hydrogen and that hydrogen is connected to the other carbon which that is crazy.
do your videos have any order and organization? I don't want to jump from one video to the next.
THANKYOU LIFESAVER!!!!
Have an Ochem exam tomorrow....wish me luck
good luck bud
have my natural products test on Friday haha
So, what type of nucleophile will determine if it will be a SN1 reaction or a SN2 reaction?
so helpful!
@7:48- how do we know if the Nu: attacks from the front or back?? I know that you said it should attack from the back and why, but is that always the case? Can you explain this further? Should it always attack from the back WBR the Nu: is a Br-?
Any practice question for reaction with formic acid?
Hello
How do you know what's front and what's back? Isn't that relative?
Thanks
Why does the oxygen develop a partial positive charge instead of negative when bonding to the carbocation?
U said that "since bromide repels oxygen ....inverted product will be formed"....but this is Sn1 reaction so the leaving group have already expelled ...then there will be no repulsion for oxygen right ??? Kindly answer
The channel's name says it, make sure to add a "best" in front of the existing channel's name though.
What else could replace this method ? -In case it is needed.
Thank you so much
I don't get how the bromide affects the water if it attacks from the front of the molecule, but not the back? It's all in the same solution, right? So, why can't the bromide affect the water if it's attacking from the back?
It's not really about "front" and "back", but about "one side of the plane" and "another side of the plane". That carbocation is sp²-hybridized, so its geometry is trigonal planar, and normally the water molecule could attack it from both sides. But the negative bromide ion is still hanging around nearby, attracted by that positive carbocation, and partially blocking the way from one side for that water molecule. Therefore, that water molecule has still a better chance of attacking the carbocation from one side (the one without the bromide ion) than the other (where the bromide is still hanging around and blocking its way).
i think it should be the nu- to take the h rather than h2o
Ultimately it will. But if there's a + charge on an atom, it would want to get rid of that charge by losing a proton to the nearest neighbour that can take it, which might as well be some water molecule nearby. Then this water (now hydronium ion) can give that proton to another water molecule, and the proton may change its owner for a while until it reaches a better nucleophile with a negative charge that could accomodate it better.
What I would be worried about more, is that in acidic conditions, it's rather unlikely to have negative formal charges on atoms :q So either this bromide ion wouldn't leave to begin with, or we're not in acidic conditions and this proton can't be given out to water. So yeah, there's definitely something fishy going on with his electron-pushing arrows :q
Wish me luck on my Chem HL exam tomorrow :(
Isn’t iodide weaker than bromide making it unable to remove it?
How will you know a reaction is an SN1 or an SN2 reaction
Orgo final tomorrow, pain
What type of stereoisomers are the molecules in the last example?
Did you mean to say Sn2 at 1:46?
I think so
I am pretty sure the first example is a SN2 example?
I- will behave differently in sn1 vs SN2 reaction
Could I have the references/source please?
hello, from Andino's class
01:41 Dear Princess Celestia, today I learned that Iodide is a naughty backstabber :J (it's also a good nucleophile, they say)
06:02 What do you mean "leaves"? It can't just break that bond, withdraw its electrons and leave all by itself, can it? It it's better off alone, why would it form a bond with that carbon to begin with? :q
08:18 Is there any way to separate those two products from the mixture?
god bless you
In sn1 what causes the bromine to detach?
Good question :q
we are performing a nucleophilic reaction which means replacing the nucleophile ....in this case its bromine :)....replying after 1yr haha