Chemical Equilibrium Explained | Video Tutorial | Crash Chemistry Academy
ฝัง
- เผยแพร่เมื่อ 14 ก.ค. 2013
- What are the hallmarks of equilibrium, what sort of processes can reach equilibrium and under what conditions, and how can equilibria be represented and interpreted graphically
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
Check out our best lessons:
- Solution Stoichiometry Tutorial: How to use Molarity
- Stoichiometry
- Quantum Numbers
- Rutherford's Gold Foil Experiment, Explained
- Covalent Bonding Tutorial: Covalent vs. Ionic bonds
- Metallic Bonding and Metallic Properties Explained: Electron Sea Model
- Effective Nuclear Charge, Shielding, and Periodic Properties
- Electron Configuration Tutorial + How to Derive Configurations from Periodic Table
- Orbitals, the Basics: Atomic Orbital Tutorial - probability, shapes, energy
- Metric Prefix Conversions Tutorial
- Gas Law Practice Problems: Boyle's Law, Charles Law, Gay Lussac's, Combined Gas Law
-More on Chemical Equilibrium | Wiki-
"In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time.[1] Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.[2][3] ...
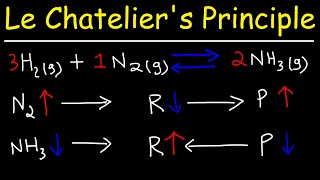
The concept of chemical equilibrium was developed after Berthollet (1803) found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions are equal. In the following chemical equation with arrows pointing both ways to indicate equilibrium, A and B are reactant chemical species, S and T are product species, and α, β, σ, and τ are the stoichiometric coefficients of the respective reactants and products:
α A + β B ⇌ σ S + τ T
The equilibrium concentration position of a reaction is said to lie "far to the right" if, at equilibrium, nearly all the reactants are consumed. Conversely the equilibrium position is said to be "far to the left" if hardly any product is formed from the reactants.
...
By convention the products form the numerator. However, the law of mass action is valid only for concerted one-step reactions that proceed through a single transition state and is not valid in general because rate equations do not, in general, follow the stoichiometry of the reaction as Guldberg and Waage had proposed (see, for example, nucleophilic aliphatic substitution by SN1 or reaction of hydrogen and bromine to form hydrogen bromide). Equality of forward and backward reaction rates, however, is a necessary condition for chemical equilibrium, though it is not sufficient to explain why equilibrium occurs.
Despite the failure of this derivation, the equilibrium constant for a reaction is indeed a constant, independent of the activities of the various species involved, though it does depend on temperature as observed by the van 't Hoff equation. Adding a catalyst will affect both the forward reaction and the reverse reaction in the same way and will not have an effect on the equilibrium constant. The catalyst will speed up both reactions thereby increasing the speed at which equilibrium is reached.[2][4]
...
Although the macroscopic equilibrium concentrations are constant in time, reactions do occur at the molecular level. For example, in the case of acetic acid dissolved in water and forming acetate and hydronium ions,
CH3CO2H + H2O ⇌ CH
3CO−
2 + H3O+
a proton may hop from one molecule of acetic acid on to a water molecule and then on to an acetate anion to form another molecule of acetic acid and leaving the number of acetic acid molecules unchanged. This is an example of dynamic equilibrium. Equilibria, like the rest of thermodynamics, are statistical phenomena, averages of microscopic behavior.
Le Châtelier's principle (1884) gives an idea of the behavior of an equilibrium system when changes to its reaction conditions occur. If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to partially reverse the change. For example, adding more S from the outside will cause an excess of products, and the system will try to counteract this by increasing the reverse reaction and pushing the equilibrium point backward (though the equilibrium constant will stay the same)... ."
Wikipedia contributors. "Chemical equilibrium." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 30 May. 2016. Web. 3 Jul. 2016.







After three whole years of study..I just now learnt chemical equilibrium in real sense.
I have yet to find a video as clear and as well structured as this, thank you!!
Thanks much!
GREAT intro to the topic-- nice explanation of the concepts and graphs
perfect intro. thank you!
Thank you for making this topic interesting
you help me brow..you made this topic easy for me
I love how you present the particles on how they met and reach equilibrium. Also the graphs. Its clear. Thank you for this❤️
Thank you!
Wish if you taught me in high school or university! Clear and structured lecture! Hope professors can learn from you
I'm in 2020 quarantine but it helped me a lot
An excellent way of explaining equilibrium reactions. This really helped me in further understanding what my teacher was teaching me today in year 12 HSC chemistry
I'm glad it helped!
Beautiful. I get it. Thanks.
Perfect explanation doesn't exi...
After months of struggling with this topic I found your amazing video and I can't believe I am saying this but I feel like I finally understand how equilibrium looks
Awesome! Thank you so much!
thanks a lot.....
Thank you sir (Great teacher)$$
You're welcome!
I just created a playlist titled 'amazing explanation videos' to put this in, not even joking.
Love it! Can you give me the link?
My teacher sent me here 😄
hero