Trick To Draw Lewis Dot Structures
ฝัง
- เผยแพร่เมื่อ 8 ก.ย. 2024
- This video shows you how to draw Lewis Dot structure in 5 easy steps.
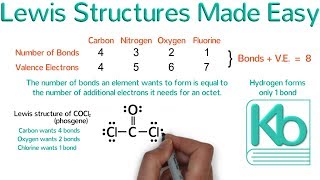
1. Count total valence electrons for the molecule.
2. Put the least electronegative atom in the center with other atom spaced evenly arount it.
3. Draw single bond to the central atom.
4. Complete octets on outside atoms and add remaining electrons (If any) to the central atom.
5. If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
If you want to learn entire Chemistry in very short time with all clear concepts and guaranteed marks, whatsapp now @9110662880.






![[LIVE] MCHOICE & MINT AWARDS 2024 at TRUE ICON HALL, ICONSIAM](http://i.ytimg.com/vi/wMewUeT5nbY/mqdefault.jpg)

Even after 7 years you are helping us a lot those who are watching it after 7-10 years please like 👍
In just six minutes you explained it well !
Thanks God for I got such a great TH-cam channel !
The thing you explained in 6 min was way better then my 6 month of understanding this
WOW I love your left handed writing and your explanations are clear enough to understand
Only you helped me to learn this 🙏🙏🙏🙏🙏🙏
Bhai physics wallah ke video dekh isse tuzhe kuch bhi solve bhi hoga ye mam ne aadha knowledge diya hai
@@chaitanya.gatade2080 there are lot in hindi language but by using simple english lang there are very few
@@chaitanya.gatade2080 very well said bro alakh sir taught best.
@@chaitanya.gatade2080 physics Wallah students are so toxic
😂
Very good video..was very helpful. Thank you. For those of you who mentioned that N is in group 15, just so that you know it can also be called group VA, hence 5 valence electrons. Kindly do check the latest periodic table charts available. Secondly, language should not be a barrier for sharing your knowledge with someone as long as you understand the concept.
Nisha Alex
👏👏
🌿🌷nice re!
Same idea as yours
Yes in iupac name it is 15 group but normally iv group
Ma'am your explanation is far better than our teachers... I wish you be our teacher... we have TOPED in the exams....
THANK YOU
I'm in 11th and confused about this but know it is crystal clear 🙂🙂
bruh ur english
@@mrbrassballs7704 its correct
@@Anoxyy_7 oop
@@Anoxyy_7 almost correct
Thank you so much it helped a lot to me you have saved me from my lecturer
You teaching has made me very strong in Chemistry Ma'am!! Have no words to express!! A trillion thanks to you!!
Thank you mam you literally make it very easy for me to understand. Once again thank you..
Who came after watching alakh pandey
Mam no words to thank you . I love chemistry because of you and I am very confident that I will score more marks in upcoming NEET examination . All credit goes to you . Love you Mam.
17th coming soon
Everyone knows
How does your neet went?
Simps
@@devanshvikram6688 Did*
Mam, pls explain how to draw Lewis dot structure for N2O
•• ••
N•••-----•••N•----•O••
••
Thank you, all your videos are very nice esp. aldol condensation. You made it super easy for me
Jis bhi teacher se samjh a jaye samjho wahi best hai appke liye ...
Thanks mam I got all the point ....
Tomorrow is my test mam. And your this vedio helps me very much
That was indeed very helpful .A big Thank You Ma'am .You are doing a great job👍🏻
Thanks Akka
Thank you mam it's been too easy to do I have never seen this type of teaching
Not applicable on all structures which are more appropriate.
Will not help in any big exam like jee,neet etc. because they give questions based on resonance
@kashish tiwari ha pta hai yrr isko dekh kr Vahi gya tha
Theivamaee..... ithana naall...unagala eappadii miss panna.... chemistry semmaiya eadukuringalaeee....❤ I think u may clear my fear in chemistry 😊❤❤
how to find out whether the molecule is lewis acid or not.. like PCl5
Molecule with incomplete octet
EX. BeCl2 BF3 etc are Lewis acid
Mam u r providing a very better way to children.. Itz really vry helpful ☺☺☺
Amazing
Thank you very very much for this mam
But can you explain for SO3 PLSSS
Mam your tricks are just amazing 😃😃
Thank you so much maam u clarified my doubt by doing this video
I am in first semester and our chemistry teacher takes one hour to teach this concept but whole class unable to clear there concept but saw these videos and i cleared my concept with in six minutes and also share to my friends.Thank you ma'am and continues to make such videos
I fall in love with chemistry bcz of u
Please , add the how to find lone pair in +1 chemistry please
Watch physics wallah videos
😂😂😂. Yes they are joking Lewis dot structure 6 min. Mai ho hi ni sakta😂😂😂
Sahi kehthe hai hmare sir :Alakh pandey g
True😂😂
1 ghante ka kam se kam chiye
Yeah bro yeah of course😂😂
He boring lol
I am watching from Bangladesh, ma'am, the class is amazing🇧🇩
Ma'am, could you please give some more examples in another video, please Ma'am? You need not even explain this much, but just some more polyatomic ions Ma'am............
Thank you so much
Very clear teaching 😇🤩🙂
"2. Put the least electronegative atom in the center with other atom spaced evenly arount it."
I don't think that would be a general rule, because what about H2O, where you put O in the center? Or SH2 where S is in the center?
The only exception to this rule is that Hydrogen and fluorine can never be the central atoms
@@carry4093 you sure?
@@divishg4428 yes atleast in the jee syllabus
For choosing central atoms there are many things to consider, a) least electronegative atom; b) least number of atom; c) Hydrogen and Fluorine cannot be in center as hydrogen is monovalent
Mam this is all bcoz of you I am able to see much difference in my previous marks comparing to now
Explain core concept, applicable to all, not just one😄😄😄
Yeah right
Simple and short
Thank you mam
How can we put - charge directly there is a formula for it on which atom we shud put the charge
Nice explaination 🙏🏻👍🏻👍🏻👍🏻
Mam ur teaching is excellent !! Now I am getting more confidence !! I easily solved many problems of chemistry !! Tq u so much mam 😊
Its really very helpful mam..
Thnq so mch
Really greatful of u..
Wow Teacher ur the best Teacher in the world ❤️ Thank you so much for the service ur providing for students like ur....God bless you...
Understand from Mizoram ❤️
Thank u so much, you're a amazing teacher
Wow... Really Nice... Ma'am
Please send me number maam
Thanks mam
Cool and simply tricks 🙂 mam
😊thanks mam... Nice trick
Super mam wonder ful explanation
Luv u mam ....such a nice trick.. tricks madha pls make video on jipmer syllabus and cut off percentile.. pls 🙏🙏🙏🙏
Wow mam I never saw a great chemistry mentor like you mam what a great and simple explanation 😍 after watching this video I feel like flying ❤️
Ever after 4 years the comment section is live
Concept is Crystal clear 😎🥰❤️
Thank U sir 🙏🙏
😂😂
Than you so much mam. This is very helpful video for me
Mam strong field ligand are poor Lewis acid or Lewis base?
Thank u maam ❤
Thanks mam by sole of my heart. For help
Gracious mam ❤
Thanx a lot mam
Thank you..mam for rhis video so easy explain you..very helpful for me
Mam I am subscribed ur channel and your trick was amazing
Good English nice going
Nice mam thanks 👍
Mam since there is minus charge above the ion we included +1 in no of electron then why are we putting them in bracket at atlast
To show that it has negative charge
From u video I understand clearly
Thanks mam....u r amazing teacher !! 😄😄
Thank you ma'am ❤
❤
You helped me understand the octet rule!! Thank you so much!🙏
This procedure will be wrong for the PO4 three minus. We should also use the Formal charge in the process to get the accurate answer.
Gd Morning madam. Please say how can find "BOND ORDER" for molecules which contains "MORE THAN 20" Electrons.
It was really helpful for all .. thanks
Thank u so much ma'm..I wasn't able to understand it but after watching ur video it became very easy for me..
doubt-oxygen has 6 valence electron but u have added an extra electron to make a single bond with nitrogen
It gains one electron...know see the qn..it gains one elec..thst s y we call it nitrate ion
Plz do videos of some expectational compounds in lewis dot structure..
Thnkyou so much mam this helped me alot ❤️
Thank you ma'am
Very nice 👌👍👌👍👌👌👍 👌👌👌👌👌
Thankyou ma'am..This helped me..Will you please upload a video on how to write the product in organic eqautions and also upload a video on mechanism of organic reactions(class 12 cbse)
Thank u so much mammmmm
yashodhar ur correct
Grest mam 🤗🙌
Mam pls upload video on trick to find covalancy. Plssss mam it’s a request
Hi ma'am please reply......... How to find oxidation number from Lewis dot structure...... How to make and find oxidation number.
Alakh sir 5 min video ki talash me kaun kaun aya hai
Me😂
Why are physics Wallah students so toxic?? Ew
Are bhai sach me
Me bhi usi ki talash me hu
Thankuu mam, its very helpful
Thank you mam. You are a amazing teacher.
Nice mam
Ma'am u r awesome but please you have to give some more examples because your trick is very simple and fantastic I really like this ...
..thanks and try to make some more vdo in chemistry...
Madam,it is given in books that co2is an octet deficiency molecule..but as per lewis st of co2,all the atoms fulfil the octet configuration..will pl clarify
Superb...... Very helpful and also easy to understand!! Keep on uploading similar videos. Thank you.
Really great mam 👏
Hllo komali mam plzz gives some more example to clear concept
Thankyou ma'am
I know Nitrogen belongs to 15th group but Mam first says about valence electrons
You clear my concept thank you so much madam
Smtime thats eg things are out of mind...tq .Fo remembered❤️❤️
Thanks Mam Really helped .
what about structure of H2SO4.....in that S is making 6 bonds end exceeding its octet
saksham semwal sulphur can violate the octet rule
Thats called hypervalent atom
Limitition of octet rule.
Splendid work mam
Thanks lot lot and lot for this
Tejinder Kour
Hi
Thank you mam
mam u are left-handed? ?
Thanks mam you cleard my doubts.....
And i love your voice ❤❤😊😊😊