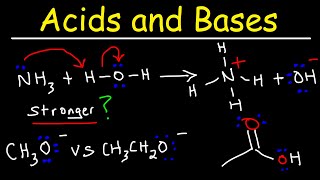
Acid Base Strength - Which Is Stronger?
ฝัง
- เผยแพร่เมื่อ 20 ต.ค. 2024
- This acids and bases chemistry video provides a basic introduction into acid strength and base strength. It explains how to determine which acid is stronger given the Ka and PKa values of the acid. It also explains how to determine which acid is stronger by considering factors such as size, electronegativity, and stabilization of the conjugate base. In addition, it mentions how to determine which conjugate base is stronger based on the Ka of the conjugate acid. This video contains plenty of examples and practice problems.
Acids-Bases - Free Formula Sheet:
bit.ly/3NsE7aP
________________________________
Acids and Bases - Introduction:
• Acids and Bases - Basi...
The 7 Strong Acids to Memorize:
• How To Memorize The St...
Conjugate Acid-Base Pairs:
• Conjugate Acid Base Pa...
pH and pOH Calculations:
• pH, pOH, H3O+, OH-, Kw...
Estimate The pH Without a Calculator:
• How To Calculate The p...
_______________________________
Autoionization of Water - Kw:
• AutoIonization of Wate...
Which Acid Is Stronger?
• Acid Base Strength - W...
Acidic, Basic, & Neutral Salts:
• Acidic, Basic, and Neu...
pH of Weak Acids:
• pH of Weak Acids and B...
Buffer Solutions:
• Buffer Solutions
_________________________________
Polyprotic Acid Base Equilibria:
• Polyprotic Acid Base E...
Acid Base Titration Curves:
• Acid Base Titration Cu...
Acids and Bases - Practice Test:
• Acids and Bases Review...
Ksp - Molar Solubility & Ice Tables:
• Ksp - Molar Solubility...
Complex Ion Equilibria:
• Complex Ion Equilibria...
___________________________________
Gibbs Free Energy, Entropy & Enthalpy:
• Gibbs Free Energy - En...
Entropy - 2nd Law of Thermodynamics:
• Entropy - 2nd Law of T...
Electrochemistry Practice Problems:
• Electrochemistry Pract...
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tuto...








Acids-Bases - Free Formula Sheet: bit.ly/3NsE7aP
Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Full-Length Videos & Exams: www.patreon.com/MathScienceTutor/collections
Next Video: th-cam.com/video/-6Pxr7SRgeo/w-d-xo.html
i love how calmly you explain everything, it all makes so much logical sense thank you so much :)
Always saving me before an exam...THANK YOU!
Please continue to make videos, you have made changes to many people's lives! Thank you very MUCH!
amazing video, if I keep grinding this sem I should finish w a perfect 4.0 for 2nd sem in a row or worst comes to worst 3.92 overall, ur videos and chads prep are saving me so much thank you from a future dentist, ur a great human god bless
your such amazing teacher please dont stop making these chemistry videos
Never knew wizards needed chemistry
Endermeen 2001 X'D we do magic involve chemistry
HAHAHAHAHAHAHAHA
I'm gonna be tutor for general chemistry this coming fall semester and I'm going reviewing all the topics in GC. This video was great for practice. Thanks man
If someone were to ask me right now who should get 100m subscribe first I choose your channel. All your calculus, chemistry and physics videos are huge help.
Thank you so much! Excellent video, you are a great teacher, you made a difficult concept very easy to understand!
this video is condensed extremely well. thank you!!!
thanks so much, helped me right before my chem 104 exam
You deserve a god damn medal, sir. Thank you
Hello tutor, I can't begin to explain you how much your videos are helpfull, so thank you ! I just had a request, could you summarise all the concepts you overviewd at the end of each vidéo, just to Make it clearer and give us a structure. Thank you in advance. #belgium
Heyy this is a great video! Although I have a question. What if we aren't given a Ka value for the first question. Is there a way to identify which acid is stronger?
I really was googling this as well.
th-cam.com/video/v_94NsuBwDM/w-d-xo.html this helped me if anyone is still looking for a similar answer
What if Ka is not given?!!!!
There are certain trends for H-X acids with X being a very electronegative element, as well as H-O-X acids and H-(n)*O-X acids you just gotta memorize
Rick F lol, that’s not chemistry, it should compare stability and electronegativity
@@alexp2019 yeah that's what he's talking about in terms of trends
well, u remember the strong acids and the strong bases, then you know also bases and acids that have zero strength. The rest are weak acids, or base and to find out which one is a base or an acid: see which one gives protons and receive protons.
he goes over it in the video--
u are really good at explaining things easily please keep it up
Jesus Christ, the way you explain is perfect, thank you for the videos
this was AMAZING! Thank you so much
I actually didn't realize when it had just ended.....very nice video... I totally lost in it 😲😰😰
When you accidentally enjoy science haha
1:50: important detail about oxy acids is that you need to look at the unprotonized O-Atoms in the molecule, so the actual number to estimate the Ka value is [number of O-Atoms in the molecule] - [number of H-Atoms in the molecule]
also you can roughly calculate the Ka value just by that number. just multiply the number of unprotonized O-atoms by 5 and subtract it from 8.
The fact that French is a my first language but I understand better in English 💀 you literally the goat tyyysm
You are a lifesaver for this
Wow🎉,ur lessons are the best🫡🫡
i wish you explained more, thank you though
Amazing tutor
👌 love from INDIA🇮🇳🇮🇳🇮🇳🇮🇳🇮🇳
I managed to get the first 8 questions correct.
8:01 True for *inorganic* acids; organic derivatives of these acids can also be strong, such as methanesulphonic acid.
Thank you very much. This is excellent.
which is weak?
HMnO4 or HNO3
And please give me the explanation. Thank You😊
HNO3
Very helpful .Thank you so much .
Thank you very much i really know now how i can Distinguish
Great work sir. Thank you so much
How many indians love ❤️ this channel and him
I was once confused because of absolute values, when here, the bigger the negative the smaller the number.
How we find Ka and Kb values??
What if I have to rate from strongest to weakest, but I have HCl, H2SO4, HBr, HNO3, CH3COOH? How do the trends work if I have both acid with and without O
Thank you
What about ranking these: water, nitrate, acetate (in ascending base strength)?
which acid is stronger sulfuric acid or Nitric acid
Thank you so much
For number 7 at 9:10 are HS- and NO2- the conjugate bases?
Yep!
Thank you very much....
You are amazing, and your voice is so nice lol.. could be a good ASMR voice to help me sleep too.
what a lovely vedio;👍👍👍👌👌❤💙💟 from india ;kanpur
That is amazing video
Thanks
Gonna name my firstborn in your honor
Ty
THANK YOU
Ur teaching is awesomesauce
Perfect
How do you know the Kb value??
for HIO and HBrO wouldnt the stronger acid still be HIO because the hydrogen is technically attached to the I not the oxygen??
I thought the same thing. Contradicted himself.
8:57 what if there's no ka or kb
What about H2SO4 and HNO3?
so helpful ong
Why Hf is the weakest acid ??
Because it's the toppest on a group so it's size will be small as compared to HCl, HBr, HI. It will be strongly bond to the hydrogen atom and thus it will not loose it's H+ easily which makes it a weak acid. Those below the group have weak overlaps because of large size of the X atom so they'll loose their H+ easily making them a strong acid.
What if ka is not given
You rock
What is the reason behind 6th question? Becoz I know HF is stronger than HCl due to it's high electronegativity
It's actually the opposite. HCl is one of the strong acids, HF isn't. The high electronegativity of fluoride holds on to the hydrogen more strongly, so that proton is less likely to dissociate -> its a weaker acid
I agree with BledWh1te. The strength of the acid could also be determined qualitatively by considering the relative stability of their respective conjugate bases. Using this perspective, HCl would be considered a stronger acid because Cl- would be more stable than F-. In this case, the larger the atom the more volume available to disperse the negative charge. This trend continues down the same period. F- < Cl- < Br- < I-. Hope this helps!
Cl is bigger than F so the bond between Cl-H is longer than the bond between F-H, and we know the longer the bond the weaker the bond so Cl lets go of it's Hydrogen much easier, and acidity is how readily an atom gives up its proton.
Higher electronegativity only wins when going across a row (moving left to right). But when in the same column/group, electronegativity doesn't win, atomic size does. So going down a group/column means greater acidity despite electronegativity becoming smaller. Reason: bigger atom=more surface area=spreads out negative charge better=more stable=more okay with letting go of that hydrogen.
you are a god among men
Sbf5
If I had million dollars I would give them to you .
Konjam sound ah pesunga plzz
R u here for your competitive exam?
Add lyrics please
strengff
HF is a weak acid lol.
HF is stronger than HCl
If the weaker living are destroyed and vanished, what good and bad values go vanish with them. If we have the forced will to destroy the weak, we find a good reason. We had less emotional intelligence. Or i couldnt stand her dumb mind. Did she have got any strong values. She was dumb. In a good way. She saw equality in all living. She was good, best, bad and worst in the same person. She saw herself in all living. She was a weaker person. She was a living in this world, where all living are the same.
Sale sare paise kamate hai
Koi free mein padhata he nahi
Doesn't explain anything, just memorizing information... sad
There's not much to explain. This is a really easy concept. Be glad he's even making a video about it.
Yesss exactly I wanted an in depth video (˘・_・˘)
Thank you