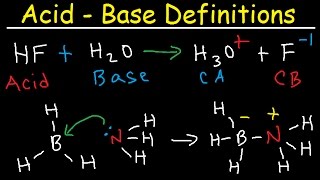
Acids and Bases, pH and pOH
ฝัง
- เผยแพร่เมื่อ 10 ม.ค. 2016
- We've all heard the terms acid and base. What do these mean? Don't just tell me about pH, silly. What structural detail makes a molecule an acid or a base? You don't know? Well, you'd better watch this then. You don't want to be embarrassed at the water cooler.
Watch the whole General Chemistry playlist: bit.ly/ProfDaveGenChem
More AP Chemistry review materials from me: bit.ly/URPDave
Organic Chemistry Tutorials: bit.ly/ProfDaveOrgChem
Biochemistry Tutorials: bit.ly/ProfDaveBiochem
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDavePhysics1
Modern Physics Tutorials: bit.ly/ProfDavePhysics2
Mathematics Tutorials: bit.ly/ProfDaveMaths
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT








In 3 5-10min videos (approximately 30min max), you have successfully taught everything that my professor taught over 210 slides in a month. You are a blessing Dave!!!
So far I've had 2 Chem teachers for intro chem to science majors. My first teacher had ZERO language precision and my second teacher talks waaaaay too much and takes way too long to explain everything/anything. Professor Dave is very precise with his language and very concise with his content. In a world where Tik Tok, Insta, Reddit and Facebook ruin your attention span the hero we always needed is Professor Dave.
If you ever read these comments I want you to know: You're gonna do a lot of good in this world with your videos Professor Dave. I will petition my college to have you come as our commencement speaker once Covid Season is past us. Keep at it. You're the real MVP.
Haha I would totally do a commencement speech!
As a person with normal attention span in the social media era I can confirm this is indeed very straightforward explanation
But to be very straightforward it means you got to cut off a lot of information that may be important to know, it's not an issue for teaching the basics of the concept though. That's why I don't think there is such thing as a "better teacher" each person has unique approach to teach.
How's your program? Given the timeframe I'm assuming you're either on break or Year3 of your major!
Been saving my grade since g12. I'm already on my 2nd year college. You doing good man.
1. Thank you for not dressing up like a nerd (or not dressing up) like many other science tutorial videos out there. It's insulting to us in the science world.
2. I'm a senior chemical engineering student, and I watch your videos all the time. It's amazing how coming back to basics is essential to understanding more complex material. I'd even argue the basics are the more difficult to learn.
3. Thanks for your contribution to teaching science in a way that anyone can understand. Your summaries frankly cover the main points of entire courses I've taken (though I appreciate that studying and practicing the details is very important, too).
"I'd even argue the basics are the more difficult to learn." More on this? interesting thought
@@NewWesternFront thats not an argument its a plain fact.
@@cabbage5114 what makes the basics more difficult to learn?
@@NewWesternFront you have to take the information and believe it regardless because its true. Then you will have to apply it to everything else you learn. If you dont learn the basics, you will not understand anything.
I'm here for the opening song 😂😂😂
My 3year old son sings this song
you are awesome you should know that , you teach better chem than my teacher okay
+Rutwik Pandit thanks kindly! spread the word!
Professor Dave Explains i m a big fan of urs
honestly same
It's one of the most underrated channel
You rock :D love your simplicity, clearness and how brief you are.
I literally do not understand how I did not understand this before, you explained this very well and I thank you for that.
I had been struggling with understanding conjugate acids and bases. Within the first two minutes of your video, I understood a concept that I had been struggling with for weeks! Thanks Professor Dave!
Hi professor Dave that last table suddenly made sense of everything. My life is now complete
Hey Prof Dave, I'm a pre-med student planning to take the MCAT early next year. Your videos are making the review process straightforward. Thank you for what you do!
How's it going?
This is super helpful and clarifies concepts in my text that I was not able to get my head around. thanks
You are the most helpful outside-of-class resource for Ochem that I've found, by far! I really appreciate the amount of effort you put into all of your videos. You are the bomb!
Thanks for giving us short topic videos it's helping me much as I didn't expected and also you are better than my chemistry teacher
Very clearly explained. Thank you professor ❤❤
thank you so much pls know what u do is sooooo helpful idk where i would be without these
I liked the way you explain and communicate the information👏💜
Your awesome for review I shared you to my teacher
Thank you so much!! This was one of the only sources I could find that explained how to predict the stability of a conjugate base using atomic structure. I knew there had to be something that determined it, but most sources I found just said to memorize the list of strong acids -_-
You are the best human to pass on information !!! as a teacher you get an A+ , thank you so much for your videos ...
This videos is great Professor..
And this educative videos is consistent,,
Should be more subscriber..
i agree, please tell your friends to subscribe! :)
Thank you Prof.Dave this has helped me a lot
This video clears most of my concepts on ionic equilibrium
These days all what I watch on TH-cam is your vids
Hopefully I'll pass my biochem exam
Amazing summary. Thank you
Thanks for getting straight to the facts ‼️
U kind of help me with All my confusions. Thanks
Very great animated explanation.
Sir I love your channel and I am your big, big, big, big fan
Thx sir for making chemistry more easy to understand 😃
Thank You Professor Dave!
Thanks for this wonderful video
Thank you Dr. Dave
thanks sir for a great explanation...
my boy professor dave you now you are a lifesaver
Phenomenally explained!
honestly amazing. 10 min video, made 7 pages of notes from it!
This guy teaches better than the public school that my teacher has us watch him for lessons. Tells you a lot about our education system.
Bad professor with the buttons down lol. Bless you Dave you're doing gods work!
Thank you Professor Dave!!
6:24 is it "weak acid ...generating some conjugate base instead of conjugate acid?"
I LOVE your Channel , I keep rewatching it for revision , Love u professor
hey dave ur great
You're just awesome💜💜💜, I really hate chemistry but this is the first time I'm questioning myself can chemistry ever be so interesting?😂
You r great sir.....thanku
you are awesome!
Love it it really helped
Thank you so much 🥰
Very useful! Thanks! :) I really like the chart in particular
good stuff
Thankyou sir
❤️❤️
So just to clarify, at 4:48, it is predominantly due the molecular geometry, and its effect on the molecule's polarity, that H2O is much more inclined to donate a proton than CH4? And when water donates a proton, how, or would it effect its electron distribution among its orbitals (for example, s&p orbitals)?
no, very little to do with geometry, it's simply that an oxygen atom is so much more capable of accommodating a negative charge than a carbon is, due to its electronegativity. after donating a proton, the lone pair left behind will occupy the same orbital it did beforehand.
When calculating acid constants, shouldn’t activities also be used instead of concentrations? Activity coefficients close enough to 1 can be replaced with concentrations but only with smaller ion strengths.
Sir is it possible u expain these things little prolong and in detail that we get full
here in 2020-2021 school year where all my classes are online god bless this man
i watch your videos religiously, thank you professor dave!
What does that mean?🤔
You are one of the best chemistry teacher I've ever come across 😁..
Hi Professor Dave, should I memorize that chart for pOH/pH conversions?
I have a question
Is there a chemical that is considered a weak basic oxide?
Most of the basic oxide I researched is mostly strong.
Thank you for reply!
Yep pH have to know what to be a good farmer the grow anything with her organically or especially in organically what's hydroponic of soil you still have to know about pH value acid or alkaline scale as you know some may not goes from 0 to 14
Around 4:45 Professor Dave explains that an iodide ion is more stable than a flouride ion because of the difference in area for a charge to diffuse through, he then proceeds to say that also electronegativity plays a role but explains with a different set of examples. What I would like to know is how the electronegativity of iodide and flouride determine which atom is more stable?
I still find it hard to accept that the iodide ion is more stable, most atoms love to exist as ions coz it's more stable. If a flouride ion has a better hold on it's electron than iodide, plus has a larger electronegativity then how come the iodide atom is apparently more stable? Someone please clearfiy this to me, before I lose my faith in the wonderful consistency of Chemistry!
Please tell the answer of the given question?
Why aniline is more basic than ammonia in gas phase but less basic than ammonia in liquid phase?
you rock
Excellent video! Exam in couple days, and this is just what I needed. Solved the last tasks, with thanks to the help of your exceptional explanation! Now I understand much better than what my teacher could explain
Awesome sir from which country u are sir?
You are amazing 😍😍
At 4:30 why is the I- ion more stable? I mean, if it's larger I believe the electron he gained is further from the nucleus, isn't it? So it can donates the electon more easily and sooo it's a stronger conjugate base and the acide should be weaker? I know you're right ofc I just want to say I don't understand. :) Hope you can answer me fast, thank you for your videos!!
it mainly has to do with the size of the ion, iodide is much larger and can diffuse the negative charge about a greater volume, kind of like the way a formal charge is stabilized by delocalization through resonance.
Thanks!
Lich chgitein inch er. Apply sodium bicarbonate mixed with water on your hands every day. It treats a thing or two.
minute 4:00 - you say "whichever atom is losing a proton..." do you mean whichever molecule is losing a proton? As with HCl, it is not a proton integral to the Cl which is leaving, but the H from its ionic molecule?
I love the videos and how well they explain everything but its impossible to take notes without writting down almost every sentence in the video 😂
thats just how concise he is
Accept an electron = acid? Donates = base ?
It says proton but atoms don’t donate protons?
Sir I feel very sad about you because you work so hard, answer to all the questions then to your channel is subscribed by less number of people. But you continue with your good work and I will tell to my friends about your channel and to subscribe it and thanks for the damn good explanation
well i think it's growing at a decent rate, but by all means i can use all the help i can get so please do tell all your friends to subscribe!!
Very important lesson
also when solute is dissolved in solvent, shouldn't the strength between solvent-solute be stronger than solute-solute/ solvent-solvent? But in the equation HCl + H2O --> H3O + Cl , how come solute-solute which is ionic bond(HCl) is stronger than solute-solvent which is ion-dipole(H + H2O)?
HCl is not ionic, it's covalent. and when water acts as a base it forms a covalent bond to the proton as well. it sounds like you need to head back a bit earlier in the playlist and brush up on types of bonding and other such principles.
The intro is hilarious 🤣
great help...this channel is of.
Sir, if oxygen has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. From where does this one additional charge or one extra electron come? Also in the case of carbonate ion two of the oxygen atoms are single covalently bonded, so if oxygen has 6 valance electrons and it is sharing its one electron in bond formation then how does the lone pair upon bond formation of oxygen atom count upto three I mean since it has to share one of its six electrons in the bond, shouldn't it have 5 electrons. How after forming a single bond this one additional electron comes from here so that it provides the atom with a negative charge?
sir what is in lithmus paper it's change it's color ..... smthing like reaction
hi at 4:51 im new to the. concept so im a bit confused, so how does oxygen lose hydrogen faster than carbon if oxygen wants the protons because its negative; or is it because methane is more stable than water that's why methane won't lose hydrogen?
Smooth!
Thnx you
Can anyone tell me in simple definition what difference is between ionise and dissociate and when to use those words when talking about acids and bases
best professor
At 6:26, I'm confused...is it supposed to say "generating some of the conjugate acid" or "generating some of the conjugate base"....hmmmm....
Professor, Question on Kw vary on Temp. If temperature increase, Kw will also increase that is mean pH value will be reduce, right? so the boiling water would be in acid condition? or I confuse!
Kw definitely increases, because both hydronium and hydroxide concentrations increase, but because they increase by the same proportion, i believe pH will always stay neutral.
Thanks Professor. You always give the quick response. Please continue your VDO. You are great teacher.
Make a video on purification of Organic compounds :p
U r hard worker what is ur job sir
Sir i had one more doubt that can you explain what is protonation
This man is carrying me through Chem 12
0:38 dumb question: what does that mean about the Hydrogen atom itself? It's not literally just a proton, is it?
can redox reaction take place in acid and bases?
1 hour of textbook reading vs 9 mins of this... hmm I wonder what I'll choose
Professor, can you teach us about pOH, pH and pKw as a seperate lesson?
Why a separate lesson? It's all in here.
I am lost, any more clarification on this please?
Super sir
2:51 how did we get 10x10^-14
Sir why can water easily lose a proton if it participates in strong dipole dipole interactions?
not easily! water is a very weak acid.
I'm confused in the answer at 8:20 where you add pH to pOH = 14. How did you get the 14?
It's from the 10^-7 M concentrations of the two ions.
@@ProfessorDaveExplains oh-thanks.
when writing a diprotic acid reaction of sulfuric acid, H2SO4 + H2O --> SO4 + H3O, there's a missing hydrogen on the product side, so how do you write the correct equation?
you have to write two equilibria, one for the first proton and another for the second
Just curious to know conc H3O+
no, greater than
How is that???isnt it right that if the pH