Biocompatibility as a Critical Design Input
ฝัง
- เผยแพร่เมื่อ 25 มี.ค. 2024
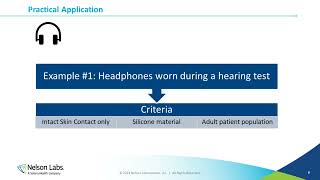
- Biocompatibility evaluation must accompany any medical device that has contact with a patient during use and is a requirement before a device can be cleared for market. This is usually the last step in device development and should be performed per ISO 10993-1. While biocompatibility should be assessed using the final finished device, it should not be considered as an after-thought, but instead be an active component of the design process.
This presentation provides an overview of what biocompatibility is, and what should be considered when performing a biological evaluation. We will also discuss the importance of choosing the appropriate materials from the beginning of the design phase to addressing potential changes caused by supply chain issues. - วิทยาศาสตร์และเทคโนโลยี