The Ideal Gas Law: Crash Course Chemistry #12
ฝัง
- เผยแพร่เมื่อ 15 มิ.ย. 2024
- Gases are everywhere, and this is good news and bad news for chemists. The good news: when they are behaving themselves, it's extremely easy to describe their behavior theoretically, experimentally, and mathematically. The bad news is they almost never behave themselves.
In this episode of Crash Course Chemistry, Hank tells how the work of some amazing thinkers combined to produce the Ideal Gas Law, how none of those people were Robert Boyle, and how the ideal gas equation allows you to find out pressure, volume, temperature, or number of moles. You'll also get a quick introduction to a few jargon-y phrases to help you sound like you know what you're talking about.
Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
Table of Contents
Ideal Gas Law Equation 0:50
Everyone But Robert Boyle 1:35
Ideal Gas Law to Figure Out Things 6:16
Jargon Fun Time 7:46
Crash Course is on Patreon! You can support us directly by signing up at / crashcourse
Want to find Crash Course elsewhere on the internet?
Facebook - / youtubecrashcourse
Twitter - / thecrashcourse
Instagram - / thecrashcourse
CC Kids: / crashcoursekids








Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
I hope we can have a Mac version too, that will be really helpful as well, but I will try it out on my phone!
A chemist froze himself at-273C. Everyone said he was crazy. But he was 0K
+Lane Messier Nice. c:
I'm sorry to ruin your joke but -273C isn't 0K
+Stop throwing fridges at me
Ice crame is best tasty on that temperatour :-D
Stop throwing fridges at me Are you sure? I thought that you added 273 to a Celsius measurement to get the Kelvin measurement.
It's actually -273.15C
We were watching this in class and the teacher pause it right after the "They almost never behave themselves," and she just looks at us and goes, "Can't imagine what that's like."
+alexiane250 Throwin shade
+alexiane250 LOL. That's perfect.
Us teachers are savage
alexiane250 omg xD
you have a great teacher
At 5:37 - decreasing the number of miles decreases the volume, but in the case of a balloon the pressure actually goes up because pressure is related to curvature (this is why it's hardest to blow up a balloon when you're starting it and then it gets easier).
The fact that this video is 5 years old and that im going to university now and still use these as an aid in studying physics goes a long way to show how well made and timeless these videos are, cheers for that
If I had a dollar for every time I had a test tomorrow and Crash Course saved my butt ...
TobyKid Major
I would be richer than Bill Gates
I’d be the owner of every cent in earth
On
I'd be eating pancakes by then
PV=nRT, or as I remember it, "PerVneRT".
You sir, are a genius.
I'd just like to say that I used this in my physics exam over xmas & got an A. Thank you.
Combat King 0 yea that what my teacher said
I love that equation...now i use it as an ammunition and shell the warmists XD
Shell the warmists? Are you really on this channel and against climate change?
On Henry Power's wiki page, it says:
"Written by Hank Green, saviour of impoverished high school children."
Well played, Hank.
hmm I don't see it
Anyone else struggling in Chem rn lmao
Me!!! I have a test tomorrow and I'm not sleeping tonight.
+Cameron Joseph Yep
I am because I have a year of work to catch up on + do another year at the same time all in six months.
Lucy Hunt Good Luck to you!
yup. watching this video the night before my final just trying g to understand lol
Hank: DO YOU FEEL IT? ARE YOU DOING THIS?!
Me: I'M DOING IT! :D
0:19 *waving my arms around*
mom: *walks in*
me:
mom:
me:
mom:
me: science *continues flapping*
mom: *walks away*
Regan Reynolds omg LoL 😂😂😂😂🤣🤣🤣🤣
@@pattys.6799 sem
This is the content I subscribe for
legit me right before i read this
😂😂😂😂
I did shake my hands around. And I did feel it
Me too! Haha.
The funny thing is I would do that when I was younger but I nor anyone else knew what I was doing
I'm really feeling it.
u feelin it now mr.krabbs?
😂 Idiots
Did anyone else wave their hands around when Hank did? XD
I did
Me too..!!
I did lol!
LOL! I did
You all are weird
Animations are done by us - Thought Café :)
Exams got me watching 46 crash course chemistry vids after finishing all of world history and economics.
life got me learning 24/7 regardless of it being the schoolyear or not faster than you study for exams
2:58 *immediately opens wikipedia and checks*
In a series of experiments with his friend, Richard Towneley, Power discovered the relationship between the pressure and volume of a gas that later became known as Boyle's law. This relationship was outlined in "Experimental Philosophy." However, many may argue nevertheless that Boyle, after discussing the theory with Towneley and reading a pre-publication manuscript of "Experimental Philosophy" cited the hypothesis as the sole work of Richard Towneley. Boyle's mention of the theory preceded the publication of "Experimental Philosophy" by one year, which, combined with Boyle's promotion of the idea and his significant status as an aristocratic scientist, ensured the theory would be known as "Boyle's Law." [5]
In eight years of chemistry I have never seen the can experiment, and that made me really happy to see something so simple but so new to me
3:05ish is where he starts talking about the science :) You're welcome. Everything else is cool too, but when studying, it can be helpful to know exactly what you need to know
Thanks
+horsecrazy2266 You're the best
EMNstar Thanks haha :)
thanks bro
Tanmay Deshpande no problem aha
This is an excellent episode of CC. Great job Hank and all of the CC team. Took me months to get my head round this at degree level. Wishing I'd seen this sooner.
I like that you broke down each portion of the ideal gas law equation. Keep up the good work :)
ppl here in 2024 🙌
I love Crash Course! Just wondering... think maybe you could make them less crashy coursey? You know, pause for breath between words now and again. I would love to see these episode lengths go up to the 22-23 minute mark so that we can allow students to have more internal processing while the video is still playing in class. ( for example, show more experiments, maps, illustrations, practical demonstrations, real world applications). It doesn't have to be Thoughtbubble (I know your contract and collaborations with them are limited and specific), but just maybe some other way of illustrating and expounding on whatever the lesson of the day is.
Just a thought. Wonderful work you and your brother do! Huge fan, keep it up!
That would be so much work and this is free education. I think this fast method is kinda cool, quick information with colors and little animations that compare things like the cation animation to help you remember if you're particularly interested you simply do your own research.
Joseph Swanner You could see khanacademy. They have a free course of Math, Science (Chemistry too!).
Change the playback speed to .75 or .5
If they did that they wouldn't need to employ teachers; we could all watch crash course lol. Works for me.
such an amazing job at teaching chemistry, keep up the great work!
I'm so glad nobody decide to enter my room when I was watching 0:20
jortjuuuuuh Porn would have been easier to explain..
jortjuuuuuh 2 11 you can see Henry powers showing a middle finger to bolye and townleey....
I am onto you CrashCourse and Thought Café
omg I love this.
He's really great at explaining things.
The energy and his enthusiasm.
I'm a fan.
thanks a lot :)
Thank you so much for this video series because it's helping me study so much and the things I memorized actually click and make sense now
This is my first time watching a video on this channel but it was really helpful and enjoyable ! Subscribed ! I absolutely love his character and his way of teaching. ♡
You have no idea how much you have helped me! You helped me pass A&P, and now in Physics! You rock, and I love your teaching style. Thanks for really helping me out!!!
Why don't you guys have physics crashcourse
See, them doing a physics crashcourse is easier said then done. They would need to teach a bunch of advance math, which not many of the viewers actually have that mathematical background knowledge.
Richard smith Then why not do a mathematics course? I'm in AAT H (Advanced Algebra Trigonometry Honors), and I would love help on that subject. I currently have a C, so I actually need it.
I would love to see that.
Vixen TheFox i would also love a mathematics crash course, not that i'm in college but i find it incredibly interesting. the thing is that math is so incredibly broad i don't know how they would manage to do a "mathematics" crash course.
Richard smith There is algebra based physics which is very simple. It's not like crash course really goes into the higher level chemistry anyways.
I love that can experiment, that's just amazing. Especially since, from what I understood, since the atom in the can slowed down so much the air outside the can just busted through. The very air you're feeling broke though metal because inside the metal was too calm.
there's also Gay-Lussac's law of pressure and temperature :) in which pressure is directly proportional to temperature (P/T = K)
Studying for my chemistry final, and I have never gotten Gases until now. My class would be so less boring with Hank Green teaching.
you can actually see the number of views for this video going up during the exam period :p
blackbirdie Cheaters be cheatin
I love the can crush experiment! I learned about in physics, of all things. How sad that college chemistry did not show me this.
Can you please make a Crash Course Physics playlist??? PLEASE!
You guys explain everything so well, and physics is one of the most confusing things ever.
2:58 thank you, you have probably helped thousands of students from that, and thank you for your videos which help millions.
Whoa this really helped me. I had been so curious about this but my teachers won't explain it to me at school. Thanks a lot!
In the midst of trying to feel the air, I bashed my elbow on the radiator
+Alisha Padaruth (AlishThePanini) you should put your hand in front of a fan
so you really did feel the gas (get it the radiator)
Finally something I haven't learned yet at high school, loved it. And I'm glad you'll go further in the next video.
Thank you for this amazing video. I have learned a lot from this episode 👍
Thanks for the jargon!
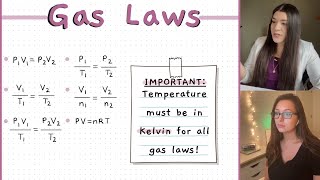
STP=0°C 100kPa
n at STP=22,4 L
ABSOLUTE ZERO=0 K=-273,15°C
Bananas=Chom choms
Actually, I believe the study of making yourself look cool while waving your hands around is called martial arts. (joke)
I really appreciate all these great chemistry videos! Thanks!
Out of every episode I've watched on TH-cam this one explains the Ideal Gas Law the best
crash couse rly has the credit for my entire education
"You can remember the Ideal Gas Equation because it looks like Pervnert."
Don't remember what cheesy-ass science video that was from, but I busted up laughing when I saw that.
I love the way you explain things intuitively to us
Thank you!!! I had no idea how is it posdible to learn everything about ideal gas law in the winter break. But now you safed my life! Or at least my chemistry grade, wich is always been A and I wanna keep it! Thank you, you're the best!
0:09
"It's everywhere!"
*lets go of balloon
*balloon releases gas
Hank in his mind: i have achieved _humor_
"You can feel it if you wave your arms around" Me: *smiles* * waves arms* *knocks my phone that I'm watching this on out of my hand*
Thank you; this episode is very helpful. Great graphics!
This was a nice recap of my school material.
I thought I've forgotten it already since I study Architecture at my university, but no I remember quite a bit.
And thanks to shows like this will not be able to forget at all. ;)
Don't be confused! The Universal Gas Constant can also be .0821 (L*atm)/(mol*K)
That's what I was taught.
Yes
That's because you're using ATM, it really depends on the units involved
Teachers be assigning this vid
This is so handy when studying and understanding the fisiology of the respiratory sistem.
I am very gratful to you Hank;
you have contributed very much in my medicine studying.
Did the can demo today in class! Then we watched a video of the same thing happening to an oil drum. Really cool.
Who went to Henry Power's Wikipedia page?
ngjo3y I did!
+ngjo3y Mee TOO!!!
I did as well!
i thougt to go but too much of work for me!
The author title gave the paragraph away. LOL
I got finals in two weeks and this is how I study for it.
same bro
I love you so much Hank. My chemistry teacher explained this for 3 hours and I didn't understand it at all.
Awesome video! Thank you for taking the time to create this and physical experiments helped a ton. Subscribing now!
when you're already studying for chemistry on the first day of school
Ap Chem problems
Im so thankful, this video helped me a lot
This is great! Helps with my summative thx hank!
ur awesomeee!!! crash course physics?
Did no one see the guy give the finger at 2:11 - 2:12?
XD
that was ur mom
I did LOL
I did, it was me. I'm famous now XDDDD *snaps and hits ground*
yup saw it
and the funny thing is, thought cafe's characters usually don't even have hands or fingers.
This was the most helpful video for my chemistry test!! Hopefully this video pays off
Love your passion of the topic.
How to calculate 1/volume(L^-1) from volume (L)
Lucy Hunt Wow
Lucy Hunt lol
Awesome.. The government should pay you for this
Loved it, thanks so much!
At my first job, I used a bead blaster to blast some aluminium. This involves a vacuum chamber with thick rubber gloves sticking into it. When I first started using it, I was very surprised at how easy it was to move my arms despite the thick rubber gloves.
Before then, I 'knew' that air was there, and that it had mass, but experiencing lack of air really changed my perception of the stuff that always surrounds us!
I love seeing the green in his hair, knowing he's using a green screen.
Amy LPS Productions he isnt
You know, I would probably find chemistry really interesting if college did not force me to learn it and use my future career and thousands of dollars in tuition as what felt like a hostage.
That makes sense. Thanks so much for explaining it all so clearly :D
Nice work giving Powers a light in history! It even says in the edit "Good Work Hank!"
why don't you post some experiment videos
The words "Jargon Fun Time" only appear on the annotation if you mouse over it,
these are so helpful thankyou for doing these
Great video!It helped me in my homework a lot.
When you have the ap chem exam in two days but still need assurance so you check crash course.
Studying for the AP Chem exam like...
+Kali Elaine same lol
Hell
in simple words, you are amazing! thanks for everything :)
I think I finally beginning to understand Chemistry, Thanks Hank :D
Fun fact! Scientist have not been able to reach 0 kelvin, they believe it may not be achievable as they believe it is really difficult to make a particle not move at all. Lowest temperature reached is around 1 kelvin.
Oh right! Thanks for the correction XD
3rd law of thermodynamics - you cannot reach absolute zero in a finite number of operations
Oh! So that's the third law, I knew of the first three but not the fourth XD Thanks.
Thumbs up if you waved your hands at 0:20...
I didn't so i dislike
dont ask for likes
This is so awesome, it tells me more detailed and fun than my University chemistry book^^
Can you make a Physics playlist please? You explain these well!
It is very impressive if you consider that air molecules are hitting the can so hard that it crushes the can.
The most valuable information I gathered from this video is that Hank's IP address is 184.166.87.227.
Hank Green the Sci Fiend, one of my favorite scientists.
Thank you for breaking down scientific theory in laymen's terms!
I remember when I first saw the crushing soda can thing- SO AWESOME.
How can they put Chinese subtitles but not Spanish??
+Fluminis Auro There are over a billion people who speak some form of Chinese as their first language, and about 400 million people who speak Spanish as a native language. Maybe that's why?
+Ningxin i know all these numbers.. but they still should make Spanish subtitles, they dont need to do one thing OR another.. anyway, i aint no bitching, im not even a spanish native speaker
+Fluminis Auro I agree, they should have more languages for subtitles. :)
I'm not sure that would work so great :/ alot of words in languages don't translate so smoothly into other languages...
+Fluminis Auro
_The Chinese subtitles aren't put up by CC._
Man, just like a submarine
Hank, love your videos. I find 'bar' is a better unit of measure when describing 10^5 Pa than using atm and having an avoidable rounding error . Overall thank you for using the metric system. :)
I was never a great science student, and I feel I only understand about a third of each of these episodes, but I still love them!
Who is watching in 2022
Yes please create a physics one!!! : ) It would be super super awesome!
I really want to thank you. My science teacher is horrible and can't explain anything at all. I have learned nothing all year but somehow managed to get good test scores (mostly by guessing). I am going to be in a much harder class next year because according to my scores, I have learned more than the rest of his students. This channel has really helped me understand at least the basic facts
i think its as cool as you do because ive done the can crushing thing for people before. also i love white monster.... ive been watching this since before work this morning and i have to tell you ive learned more now than i did in chemestry in highschool. it sucks that you werent there for me in 2005 lmao cause after trying to figure out moles. i gave up. and barely passed in highschool.
i love science and im watching ALL of ur videos, ive gotten thru biology and astronomy, and this is fantastic.
thank you.