Allotropes of Carbon || in Hindi for Class 10
ฝัง
- เผยแพร่เมื่อ 3 ต.ค. 2024
- In this Chemistry video in Hindi for Class 10 we explained what allotropes are and discussed on different allotropes of carbon.
When an element exists in two or more different forms, in the same physical state, the different forms are known as allotropes of that element.
Some allotropes of Carbon are :
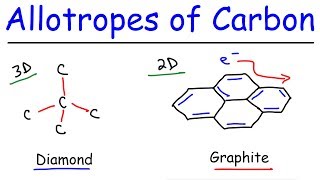
Diamond
Graphite
Fullerene
Graphene
Glassy carbon
Cyclocarbon
Some allotropes of Oxygen are :
Dioxygen
Ozone
Tetraoxygen
Octaoxygen
Both diamond and graphite are formed by carbon atoms, the difference lies in the manner in which the carbon atoms are bonded to one another. In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers one above the other.
These two different structures result in diamond and graphite having very different physical properties even though their chemical properties are the same. Diamond is the hardest substance known while graphite is smooth and slippery. Graphite is also a very good conductor of electricity unlike other non-metals that you studied in the previous Chapter.
Fullerenes form another class of carbon allotropes. The first one to be identified was C-60 which has carbon atoms arranged in the shape of a football. Since this looked like the geodesic dome designed by the US architect Buckminster Fuller, the molecule was named fullerene.
👇👇👇👇👇👇 𝑷𝑳𝑨𝒀𝑳𝑰𝑺𝑻 👇👇👇👇👇👇
🔴 Click here to watch the whole playlist on Chapter 4 : 'Carbon and its Compounds' for Class 10 :
• Carbon and its Compoun...
👇👇👇👇👇👇 𝑷𝒓𝒆𝒗𝒊𝒐𝒖𝒔 𝑽𝒊𝒅𝒆𝒐𝒔 👇👇👇👇👇👇
🔴 Introduction
• Carbon and its Compoun...
🔴 Bonding in Carbon
• Bonding in Carbon || i...








Thanks for your in depth explanation. I had so big misconception regarding its left one bond, but it is now crystal cleared. Thanks so much.
Thanks sir you are best chemistry teacher in the world
My question one carbon in the graphite is bounded to three carbon atoms but one among the bond is double bond in order to satisfy the tetravelency of carbon then how graphite have delocalised or free electrons
Two dimensional three dimensional ka matlab kya hota hai ? Sir , please clear my doubt.
Apne shayad 2d, 3d ye suna hoga 2d ka matalab 2dimensional or 3d ka matalab three dimensional
Please sir explain ncert line is ' In graphite,each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied.
3:22
Notice this view'..
Good explanation
Amazing explanation sir
Sabhi ke daily life examples bhi batana sir please
Again thanku so much
why Carbon is not diatomic? two atoms of Carbon can share their electrons right?
Nice explanation sir 👌
Now I'm studying correct subject my next board exam is chem 😀😀
thanks sir aur aise video late raho
Very knowledgeable video for me
Thanks sir,great
Wow amazing....
Thank you so much sir 😊🧐👍
❤❤❤❤❤❤❤❤❤
1 k sus challenge to
Quite informative video...
Thnk u sir..
How many corbon atoms combined to form one molecule of diamond?
Just like in cyclocarbon ,18 carbon atoms form one molecule.
Sir waha allotropes ke defination mei same physical state hoga ki different physical state sir please answer
King ho sir aap
aap he bohot aacha padhate hai
1st veiw
A great video sir
Nice session
Nice explanations...
Very nice sir.
👍👍👍👍👍👍👍👍👍
🙏🙏🙏🙏🙏🙏🙏🙏
Can you please pin me
Is there not ANY substance in earth which is HARDER than DIAMOND ?🤔
@@donotbebiased6987 o sorry! I was supposed to write DIAMOND
Bro graphene itself is harder than diamond, u seen video
Carborundum is the artificial hardest substance which I think Is harder than diamond .
Wurtzite boron nitride