The New ISO 10993-18 & Updates to Regulatory Expectations Regarding Chemistry
ฝัง
- เผยแพร่เมื่อ 3 มิ.ย. 2024
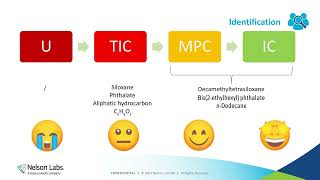
- The basic theory of how medical devices should be evaluated for biocompatibility has been in a period of flux. A cornerstone of the new ISO 10993-1:2018 is that evaluation should be focused on a rational and risk-based approach that minimizes unnecessary animal tests. A large part of that approach relies on the use of chemistry and material information to make determinations regarding biocompatibility. Changes to standards (ISO 10993-18) and regulatory policy have complicated this process. This presentation will highlight some key points in the new draft of ISO 10933-18 and provide recent examples implementing these changes in biocompatibility submissions to the FDA.
- วิทยาศาสตร์และเทคโนโลยี