Column Chromatography
ฝัง
- เผยแพร่เมื่อ 4 ก.ค. 2024
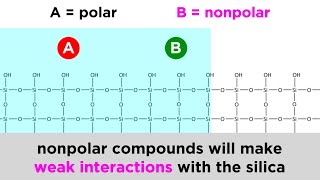
- We just learned about thin layer chromatography, which lets us separate the components of a mixture by polarity. But that just separated a tiny bit. We will need to separate entire product mixtures, so that we can isolate a target molecule. That will require column chromatography. Conceptually it's just the same as TLC, but with a column, you load everything you've got right in there, and the mixture separates as it goes down the column. Collect the relevant fractions, evaporate the solvent, and boom! Isolated target molecule. It's a little involved so let's learn how to do it!
Watch the whole Organic Chemistry playlist: bit.ly/ProfDaveOrgChem
General Chemistry Tutorials: bit.ly/ProfDaveGenChem
Biochemistry Tutorials: bit.ly/ProfDaveBiochem
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDavePhysics1
Modern Physics Tutorials: bit.ly/ProfDavePhysics2
Mathematics Tutorials: bit.ly/ProfDaveMath
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT




![ช่วยพาฉันผ่านเวลา - วสันต์17 [Official MV]](http://i.ytimg.com/vi/HFnntWoqc54/mqdefault.jpg)



THIS HELPED ME SO MUCH WITH UNDERSTANDING.. you’re literally getting me through school at this point. Thank you
First time I've actually understood Column Chromatography. Thanks a ton!
Prof. Dave always the best! I wouldn’t be able to write lab reports if it wasn’t for you
This is an amazing explanation! Please keep it up!
I love you professor Dave , you have no idea how much you've helped me
Agree
This video is literally beneficial to perceive how this process go.
so helpful.
I've watched it a few times already.
Thanks!
Great Professor Dave. after watching your videos I feel able to run column chromatography to separate compounds
Really diggin the new haircut dude!
i always come back to videos of professor Dave, such great videos
Okay. its official. i love you dave. you made my Lab report so easy and understandable.
Having to do this practical tomorrow. it will now be absolutely easy to do it
Thank you so much Professor Dave for such an amazing exploration ❤ ❤😊
Jesus H Christ! More complicated than a mission to Mars!!!😂😂
what
professor dave is an icon among stem students
your videos are so helpful! Thank you for these!
I had to double check if it was the right channel haha... nice look!
Loving your intro 0:03
Thanks a lot.
Please could you make a video about symmetry and group theory
Watching from Papua New Guinea 🇵🇬.
Thanks, Professor Dave. It was really helpful.
Thank you very much for the explanation!!!
Sorry to keep asking you questions Dave. I'm really hoping to understand this though. Assuming you happen to have a gas chromatogram or HPLC chromatogram of your sample (in some solvent), can you somehow use the information in those chromatograms to bypass the thin layer chromatography step? And would you then use the same solvent you used for the gas chromatography for the column chromatography?
What a sweet profesor you are. Thank you very much. You made me chemist
a very well done intro presentation.
if you would , please ... a few questions ... the matter of mesh size of silica gel in the
column ... for a gravity driven elution is the mesh of the TLC strip too fine to copy ?
by 'too' fine' i mean would the flow be so slow to not finish in a lab session ?
... and the fractions ... given the stopcock ... is it ok to stop the flow after each one
and spot it on a 254-tlc plate... let it dry ... then shine uV on it to see if anything
is there ? and if nothing shows up could this volume of eluent be poured back
onto the column top ?
i'm hopeful to find follow up vid's on this topic. to learn , for instance, how
one chooses mesh size of silica and the gradient solvents , plus column
size and the amount of silica vs. sample size.
Thank you very much, Prof..
The new haircut suits you
True
absolutely trueeeeeeee
Focus on studying, bro
wow amazing explanation bro thanks
great presentation. you enunciate so emphatically and clearly, good going.
this vid's been added to my favorites
one thing i'll remember is that the loaded sample has to be within the silica phase.
and not any above its top edge ... in the above sand layer.
on the topic of the sample transparent and the desired product being clear too.
does it happen that chemists add color dyes with an Rf that brackets the produce as an aid in
locating it when it exits the column ?
lastly is it ok to close the column for 10 minutes to TLC the fraction ? or does the
eluent flow have to be non-stop ?
Helps so much thank you!!!!!
Thank you so much Prof. Dave... You saved me!!!!
Thanx for the video, I've a question the compound that we obtained first , blue one 07:55 , is more non-polar, isn't it?
Thanks. You deserve a like and good comments for your videos.
thanks a lot, the same as always very nice with all of the details.
Very clear.. Thank Prof
Hi Prof. I enjoy watching ur education video. I am Hidayah from Malaysia.
Thanks for the explanation, what sand are being used at the top? what is its function? any alternatives of sand? or can it be omitted?
Sir pls tell on top sand which solvent should be added by pippte is it the solvent in which our sample is soluble????
Thank you professor!
i'm learned from video . tks you
What's solvent use to dissolve sample? Can I use the mobile phase, hexane Ethyl acetate?
Sir let me know that can we use tolune and ethanol in column .. pls tell how much sand is good in grams etc..
Thank you prof.
Sir I HV a question pls I will do this technique and I wanna ask how much would be the size of cotton swab use and for column which type of burrete is suitable???
Thanks for lot that was more helpful to me
Thank you so much you are the best!
Fourth year of pharmacy school and still benefit from you
why we should dissolve the mixture in the smallest amount of solvent? Like what happens if we add more
- Thanks
good question! so we need the sample very concentrated in a thin band so that it can separate in a distinct way. if it is dilute, then all the molecules would have different starting places as they load on to the column and you'll get very wide bands and insufficient separation.
Very nicely explained... 👌
Thank you so much!
I love now tlc and column chromatography because of you love you
"make sure to do this in a hood to avoid inhaling" 💀. I cook some other compound in the hood specifically to inhale it.
you are the best teacher eeeeevvvvveeerrrrrrrrrrrrrrr❤❤
Can you do a video on GC or HPLC please?
Thank you
Thanks you sir!!
Is it okay to use any type of sand? Or is it okay not to use sand at all? Thank you!
Looks great in that hair style
I don't find it complicated at all, but it's definitely a really clever way to the separation problem.
I like it ❤
Woah professor Dave.
Good job
Thank you.
Thanks
Thanks sir
Thanks!
What's the purpose of sand?
nice, thank you
love u prof
I appreciate u.
M also a lab trainer
The eluent is opposite to the stationary phase?
Travels through it.
Wao.
Amazing
Professor..I work for chemical reasearch industry...my question is I m working on my project which is I have to seperate two isomers spots in TLC are very close, now the problem is if I pack a coloumn of 250 gram (crude) after completion of columns I only get 100 gram pure,most of the fraction shows dual spot...that's why my yield is decreased that's why please resolve my problem,, reply me sir please
hopefully you have resolved the problem, since it was a year since the question :) I would tweak mobile phase to get those zones further away from each other
Bro why we are adding the upper layer of sand to fill the holes of silica gel or to fulfill the coloumn
it's just nice to have something to separate the stationary phase from the mobile phase, but to be honest i've run columns without the top layer of sand
@@ProfessorDaveExplains thankyou sir nicely explained
@@ProfessorDaveExplains sir im sorry to ask that again is there any holes in the silica gel. Òŕ
we are adding the sand just to fill the coloumn what is the actual usage of the sand
And im 15 years sir so i dont know more scientific terms because im tamil please explain me simply
no, the gel must be packed well, no holes or cracks.
@@ProfessorDaveExplains so the top layer of sand is to fill the coloumn
Professor Dave: what degree do u have in undergrad?
chemistry
Good
haircut took 6 years off your age
My first column is tomorrow.. Wooo
👍🏻
How is this different from gas chromatography
How much silica we have to load???
10 times the weight of mixture
Well, if I would have it done that way, they would have fired me.
First, buy a real column, not just a fat pipe. That helps keeping the gel clean (you don't want to have sand interfering with you product, or even mixing with the gel, when you unpack your column).
Second, packing your column: at the bottom should be some sort of fabric that doesn't allow the gel to go through. mix your gel with a saline or polar solution to generate the slurry. usually the gel manufacturer will tell you how to pack the column. Fill in your gel, then add the upper stamp (with fabric), make sure there are no air bubbles, and start pressing with a constant flow rate (from a pump) to pack the column.
Third: lower the stamp to the border where the gel has settled, still in constant flow. Congratulations, the column is packed.
Forth: Check the packing of your column by letting a small amout of UV-absorbing molecules through the column. You neither want to have fronting or tailing (different shapes of the peak), nor a broad peak that indicates that your column is doing a poor job at seperating.
fifth: Do the chromatography with a constant flow, some pressure and maybe even a pressure restrictor at the end of the column. And you need some sort of identification of your product, or at least a way to measure that you get different products. UV-light is very helpful, pH and conductivity are helpful aswell.
you also mentioned, that the Gel should not run dry: good advice, it usually lowers the quality of your packing. Some gels can be recovered by reverse flow, push the air out of the column. others, especially size exclusion gels are essentially done. go back and repack the column.
and there are some gels that will not tolerate air at all, meaning, that you have to throw it away, etc. A friend of mine once told me, that he had a gel worth 2 million € in his column (I don't know what it was made of, but it was experimental), and he was very cautious not to make any mistakes, because that stuff was super expensive and if it happened to be destroyed, the manufacturer of that stuff actually needed to set up a new batch in production.
You look great
New hair style...waoooo
Why does column chromatography seems so hard to me 😢
한국어로도 해주떼여 ㅎ
Just wanted to say - Love the new do! Sexy man there...😉👍
Your haiiiiiirrrr ahhhhhgg
Nice hairdo!!
finally got short hair, so sexy
You look and talk like Young Sheldon
Love from pakistan
chemistry jesus but without the hairdo
Nice haircut
Jesus cut his hair..??
Complicated
Kya baqwaas hai yeh🙂
Thank you
Thank you