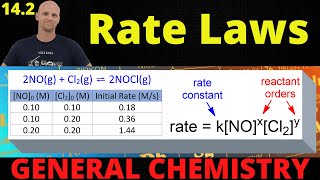
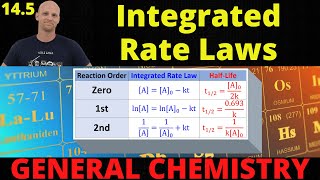
Finding Rate Law from Concentration vs Time Data
ฝัง
- เผยแพร่เมื่อ 8 ก.พ. 2025
- In this lesson, Mike discusses and strategizes how we should solve the worst kinetic problem scenario:
The one where they give is a bunch of numbers of concentration reactant vs. time and they NEVER tell us the reaction order!
Mike has a a strategy. We shall survive.







DUUUUUDE!!!!! Those first 30 seconds were my exact thoughts!!!!!! :O
thank you so much sir, i was stuck on my assignments for hours, now i got it
And if we had an addition reaction, could we determine the odder and rates in the same way?
Sure! If provided with the concentrations vs. time, it shouldn't matter which type of reaction it is. Sounds like you are currently suffering through organic 1 or 2? Most addition reactions should be bimolecular, or second-order overall, first order with respect to each reactant (i.e. nucleophile and electrophile).
Thank you, Mike! The Aleks platform and other TH-cam videos were far from helpful. It makes more sense to find the k values for two points with each integrated equation since these are constants. As well as list the y values for each order equation and look at the difference between two points to observe a constant change! My program had me calculating all of the integrated y-values and then trying to plot those by hand, and only afterward finding the k values by taking delta concentration change over delta time. I really thought this was the topic my mind was going to break and I would finally declare insanity! :)
Thank you for your comment! I tell my students at this very point it's basically the peak of the activation energy of general chemistry, part 2.
Thank you so much for making this video, + the humor
Thanks a lot for this sir , it really helped with my assignments
Kelvin 😂😂
@@shalomonyenekwe8695 😗😗
You have really helped me
Thank you very much ☺️😭❤️🥂
I have a trouble, what should i do, if i get non linear for 1st and 2nd orde? In firts trial i get 26% eror at first orde and get more than 26% eror at 2nd orde. Could i define that the orde is 1st orde ? Hopefully you see and help me to find a way 😢
homeboy needs some SPREADSHEETS ;)
Thank you!
🙂
16:11
UwU