Kinetics: Initial Rates and Integrated Rate Laws
ฝัง
- เผยแพร่เมื่อ 6 มิ.ย. 2024
- Who likes math! Oh, you don't? Maybe skip this one on kinetics. Unless you have to answer this stuff for class. Then yeah, watch this.
Watch the whole General Chemistry playlist: bit.ly/ProfDaveGenChem
More AP Chemistry review materials from me: bit.ly/URPDave
Organic Chemistry Tutorials: bit.ly/ProfDaveOrgChem
Biochemistry Tutorials: bit.ly/ProfDaveBiochem
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDavePhysics1
Modern Physics Tutorials: bit.ly/ProfDavePhysics2
Mathematics Tutorials: bit.ly/ProfDaveMaths
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT


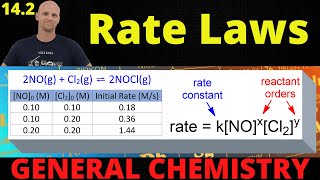






Hey everyone, TH-cam is deleting annotations, and there is an error at 5:41 that I had fixed with an annotation so unfortunately I must simply list the correction here! The values at the bottom right should read: 0.04 = k(0.01)(0.250) and k = 16/(M^2 * s), when making the video I forgot to square [NO] so please make that correction in your minds, sorry about that!
Professor Dave Explains thank you!
Sir do u mean 'top right'?
Towkir, play the video at the given time, not pause it. You'll see it on bottom right
@@Reforitor Thank you.
You're welcome bro
how did i understand this in 10 minutes but couldn’t in two weeks, honestly professor dave u out here savin lives.
Where ru from ??
@@yashasks4878 nun of u b
Definitely he's saving all of us .. I couldn't understand this from books but professor Dave made it easy 😌💙
@Jordan Polk dafuq
I know right
I have my chemistry exam in 30 minutes and you just explained literally everything I need to know in ten minutes. Can you be my chem professor instead? 😭🙏🏽
From which country u belong
i am ready
Did you pass though
If your exam was in 30 mins you wouldnt exactly have the time to type a comment I would imagine
@@ffire3795 😂
5:31
"the reaction order HAPPENS to match the stoichiometric ... but this won't always be the case"
right there, professor dave has done what no fucking textbook in the fucking world has ever done. it has pointed out something that a student might mistake, by thinking the way a student might.
hats off. well done. please write a text book or ten for students who wouldn't know any better and make that mistake. i'm a 30-something year old engineer, and i'm dead serious.
you are the man.
And i realllly like your reply u are dammm so correct
@@IbrahimKhan-lx3bm thank you. i wish i weren't.
This is exactly why I was getting my answers wrong because my teacher NOR the textbook explained that the reaction order was not in relation to the stoichiometric coefficients!!
i have a question, so the overall order is the the third order?
@@mohamadhanan5706 same doubt bro
I like Professor Dave. He tells it like it is. No messing around, pay attention type instruction in a clear and precise way. Very nice.
I agree
Can always rely on this great channel for that extra bit of explanation, much appreciated
2:30
When the concentration is doubled and the rate then also doubles, the reaction is first order with respect to that reactant. When the concentration is doubled and the rate quadruples as a result, the reaction is second order with respect to that reactant. If changes in the concentration doesn't affect the rate, the reaction is zero order with respect to that reactant.
I have a pre-lab assignment due and the instructions were hard to understand. you saved my butt by being smart and teaching the way it should be taught. Thank you !
I am a Chemistry lecturer and I find your videos very clear and concise. Well done!
Crash course: Hey so I was walking my dog on alone with my family on Christmas in the summer, and lol chemistry rate laws XD. Dave: I’m Dave here’s chemistry
Joey Santana Ikr
So you live in australia
should i be worried it took me reading this 3 times to get the joke?
Hey professor Dave!
I honestly love your channel, the writing, and editing style in [video] was a huge influence for me when I first got started!
I'm writing my Chemistry semester test today and i had thought i'd have to sacrifice this chapter but thanks to Professor Dave i have a feeling that i'm going to nail it.
Very well done video you explained it good. I suddenly understand everything now which makes me again question why im spending thousands on tuition money...
You won't get that chem engineering job without that degree.
because you have to have proof that you've been educated lol. Go to class for the rep, go to TH-cam to actually learn.
paul nicholas This is the most true statement about college I have ever read!!
Elaphe Gutatta ehh not always true. Have you guys ever heard of repetition and familiarity? I know you have. Because I think learning really comes down to that. If we watched this youtube video before lecture, im sure the lecture would have made more sense versus when you watched a youtube video about that same topic. Now this does not mean there aren’t bad professors out there because there are...but I think one reason why you get a topic more after this video is because its PROBABLY your second time hearing about it. I think all we need is to learn a topic from DIFFERENT resources to actually understand or be ‘’more familiar” with anything... Thats what books, teaching assistants, tutors, youtube videos, khan academy!, homework assignments, worksheets, online simulations, etc., are for !😄
@@brian_mcnulty you can just self employ dude, I did and now im making water treatment systems from scratch. materials and everything. no employment needed .... i I am certified by my local government. no degree needed. self taught via books, online websites, youtube. saved thousands on tuition fees. but only reason i'd go to university is if i want to be a doctor. the rest, no degree needed when self employing.
Prof. Dave, you are AMAZING! So happy I found your tutorials online & it is soo helpful!!! Thank you you're always ready to help (xoxo)
Thank you Dave. I like many first found your channel because of your debates, love those videos. But whenever I get confused about a subject during dead week and get anxious I’ll fail, you’re there to rescue me. Thank you for the great videos
When ever I see your channel I relatively find some easy ways you are like my brother giving me an easy ways to get the information thank you bro I will remember you in my entire life
Explained it better in ten minutes than my uni lecturers did in a week - thank you XD
Out here reviewing for my AP chemistry exam in May. Thank you! Very helpful!
He makes me understand the concept in just 9.10 minutes. What a great teacher. Hats off to you professor dave. 😃😃
I really want to thank you for your wonderfull video's! Everytime when I'm losing my concentration on a subject I turn to your explanations which make the subject so much more fun to learn, thankyou!
I have been struggling with this and you have I really helped thank you! I love your videos, they're always clear and helpful
Very clear quite precise presentations on various topics. The videogame presentations are well timed which Keeps concentration consistent.
I literally can't thank you enough, you make chem easy to understand
This is amazing. I didn't have any idea about Initial Rates and Integrated Rate Laws before watching the video. You are a miraculous savior. Thank you sir.
You know you are at the right place when Jesus himself teaches you chemistry
I have spent weeks on these topics alike, haven't got them through, then the professor with his majestic magic of words and examples engraves it in my long term memory
you are literally one of the few teachers out there who can teach so much with so little
love your stuff, ur saving my grades rn
thanks Professor Dave for making everything so Clear!!
Holy crap I’ve watched 4 of these videos and tried to understand but you put in such easy terms, thank you!
This stuff is super helpful for test material review. Thank you!
Excellent video with precise wording that was very clear, thank you!
Wonderful video, thanks a lot! I wasn't feeling well in class when my teacher went over this and whew it was a lot but you made it very clear in a short amount of time :)
Dude I can't thank you enough! freaking out about an exam tomorrow and this video really really helped!
This man is a legend, taught me this in 4 minutes what my lecturer couldn’t in 3 weeks
You're an actual godsend, have an exam later and after weeks of scratching my head you made it make sense in 10min. Can't thank you enough!
In the realm of academia, where complexity often obscures understanding, the illumination of knowledge is a beacon of hope. It is with profound gratitude that I extend my appreciation to the creator of the invaluable tutorial video elucidating the intricacies of rate laws. This heartfelt ode seeks to articulate the depth of my appreciation for the educational oasis they have crafted amidst the arid landscape of academic challenges.
The journey of learning is often fraught with obstacles, and the domain of chemical kinetics is no exception. With its labyrinthine equations and abstract concepts, mastering rate laws can be akin to navigating a treacherous sea. Yet, thanks to the altruistic efforts of educators like the creator of this tutorial video, this voyage becomes less daunting, and the shores of comprehension draw nearer.
The video's inception marks a pivotal moment in the quest for understanding. Its existence signifies more than mere pixels on a screen; it embodies the convergence of knowledge and generosity, serving as a testament to the transformative power of education. Through meticulous explanation and illustrative examples, the creator has bestowed upon us a gift-a beacon of clarity illuminating the path to mastery.
Every frame of the tutorial video is imbued with intentionality, each word a stepping stone towards enlightenment. It is evident that the creator's dedication transcends the bounds of obligation; it is a labor of love, fueled by a passion for fostering intellectual growth and empowering learners to conquer the formidable terrain of chemical kinetics.
Beyond the confines of the digital realm, the impact of this tutorial video reverberates through classrooms and lecture halls, enriching the educational landscape with its profundity. As students, we stand on the shoulders of giants, leveraging the wisdom imparted by educators to scale the peaks of academic achievement. The creator's contribution, therefore, extends far beyond the confines of their video-it is a catalyst for intellectual evolution and a cornerstone of scholarly excellence.
In a world besieged by uncertainty, the constancy of knowledge serves as an anchor, grounding us in the pursuit of truth. The tutorial video stands as a bastion of stability amidst the tumultuous seas of academia, offering solace to weary learners and guidance to those adrift in a sea of confusion.
To the creator of this tutorial video, I extend my sincerest gratitude. Your dedication to the dissemination of knowledge is a testament to the transformative power of education. Through your selfless efforts, you have ignited a flame of curiosity in the hearts of learners, illuminating the path to understanding and inspiring generations to come.
As the curtain falls on this humble ode, let us reflect on the profound impact of educational altruism. In the creator's act of generosity, we find hope for a brighter future-a future where the pursuit of knowledge knows no bounds, and the flames of curiosity burn eternal. To them, we owe a debt of gratitude-a debt that can only be repaid through the pursuit of knowledge and the dissemination of wisdom.
In conclusion, let us cherish the creators of educational content, for they are the unsung heroes of academia-the architects of enlightenment and the stewards of knowledge. In their tireless pursuit of educational excellence, they inspire us to reach greater heights and strive for a future where ignorance is but a distant memory.
You are simply a phenomenal teacher Dave, thanks a million !
I'm glad i didn't skip the comprehension questions, it really helped!
best videos on youtube thanks a lot! love the comprehension ending. it really makes me understand
Thank You Professor Dave!
CLUTCH
I struggled with this for 2 years. And after those 9 minutes i understand it completely. I can't believe this..
well done, i liked these videos , easy and nicely explained, thanks Prof. Dave!
Man I love the intro i just have to hear it one more time before I start learning
I can always rely on your videos to prepare me for an exam. thank you so much!
Thanks a lot professor. Awesome video 😘😘
Hi Prof Dave, I use this video in my A2 class in school. Helps a lot! Thanks!
A whole chapter explained in less than 10 minutes , thank you Professor
thanks a lot professor Dave. You are a greaaattt help!!
So helpful man, thanks professor Dave
AWESOME! ..come teach at my school! you taught me more in 9 minutes than my professor did in an hour.
god bless u. pls keep blessing us with ur videos. 2 lectures in uni were explained in less than 9 minutes. you rock!
Thank you Professor, got the basic concepts in short time period
Oh my god sir!! ❤ I don't have words to thank you ! You just saved my life
as expected professor dave, you never fail me. thanks!
It is the second video I watched of u and it is worth watching I spent a whole hour finding a good lec..but ur ten minutes sums up everything.. May Allah help uh ❤️
Thank you sir for explaining in a simple way
Thank you Dave sir my child is really very happy as she got her topics cleared because of you
Thank you so much I needed this. I was very pleased to have the correct answer at the end, letting me know that I really did learn something.
This was soooo helpful Dr Dave. Thank youuu🙏🏽❤️
Hello sir I have exam tomorrow before I didn't listen this lesson properly now I understood very well thank you and thanks for saying
Thank you so much, this made things so much easier to understand!
U did a great job explain those stuffs.. Well done
This video was extremely helpful thank you so much!!
Simple and easy to understand . Thank you !
Ex’cellent and comprehensive. Big thanks professor
Totally got it. I wish my textbook was written by you! Thanks Professor
Thankyou for your work.
Turned a 3 hour lecture and at least double that of useless practice into 10 min of cohesive tutoring. Thanks a million
Super good video, finally understand!
Very concise and well explained.....Thanks!
Fantastic explanation!
Writing my IB Higher level chem exam in 2 weeks time, thank you so much for all the content!
I'm doing standard level, not all of his stuff is applicable but most is!
Excellent explanation
thank you very much for your time and love for others sir. you just don't know what you've done for me. may God bless you
Hello professor,
For the reaction A-->B and 2A-->2B, do we get 2 different rate constants (irrespective of the reaction order)?
I've always hated kinetics cos I didn't understand them but you've actually somehow made me enjoy them and reminded me why I love chemistry! Honestly, this is by far the most helpful video I've watched on this. Its enough for someone without much knowledge but not boring when explaining the basics! :) Thank you I'm gonna pass my exam cos of you!
Thanks so much professor .You explained in good manner
You have singlehandedly saved my chem 110 grade!
You have a small mistake in 5:48, the NO should be raised to 2 and the rate constant = 16
Yessss I was waiting for someone to correct this.
u r correct
Took me a shit ton of practice problems before i got it, and understanding how to do the different problems actually helped me understand. Units are still tricky though
Excellent resource for quick revision!
Truly amazing sir....you always help me a lot
You just saved my exam.
Thank you sooo much. you are the only reason why I can pass chemistry.
0:30 - why is each change in concentration over change in time being multiplied by the reciprocal of the reactant's corresponding coefficient?
edit: and each of those expressions is equal to the rate of reaction/rate law, right?
edit 2: nevermind, you explained it perfectly after I unpaused the video to think. my only concern is to clarify if each of those expressions is equal to the rate of reaction/rate law for that reaction. right?
Great stuff professor..... So helpful
Thank you, Dave. You are the BEST!!!
To find the order, instead of remembering what Dave said, simply write down the rate law for various trials and divide the ones with same concentration of either reactant. The rate constants will cancel out and you'll have a sweet equation which can ne solved to get the value of the order of the reaction!
oh god you have NO idea how MUCH i needed this. Thanks SO MUCH.
Thanks professor Dave. This video helps a lot! :D
Outstanding clarification!
I've got my exam in a couple of minutes and this just helped me grasp the topic better..
when determining the reaction order by changing the initial concentration of the reactant, how short the time interval has to be in order to represent the true reaction rate of the reactant? thank you in advance
Thank you very much professor Dave , could you please answer how is the rate law {rate= k * [A]’ [B]” [C]”’ }derived ?
You just saved a pharmacy student from su1c1de
THANK YOU!!! I never took calculus (I did useless trig instead, regret that decision lol) so I have been scouring the internet for rate law without having had two semesters of calculus! AMAZING EXPLANATION!
Great video Dr. Dave. Do you have a video on calculating the half life of these ordered reactions?
not specifically, but i do talk about half life in my tutorial on nuclear reactions!
+Professor Dave Explains: Thanks for the reply. I'll check that one out.
literally my savior right now. thank you chemistry jesus
You’re awesome, thanks! I totally understand now!