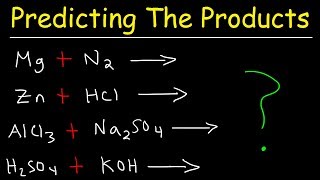
Predict the Products: Pb(NO3)2 + KI ... Lead (II) Nitrate + Potassium Iodide
ฝัง
- เผยแพร่เมื่อ 19 ก.ย. 2024
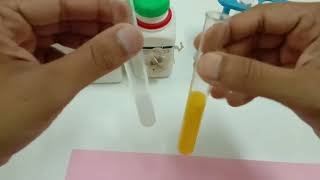
- Lead (II) nitrate reacts with Potassium iodide in a DOUBLE DISPLACEMENT reaction. PbI2, which is a solid yellow precipitate, and potassium nitrate, which remains dissolved in water.
Check me out: www.chemistnate...







From Papua New Guinea, Thank You VERY much and GOD BLESS YOU ❣️🙏
Thanks from india great work sir thanks
Class 10 chemistry ch 1 NCERT 😅
Yes bro. That's why I am here 😊
sir will you be able to help me with this problem:
when potassium iodide reacts with lead nitrate, a yellow precipitate is produced. If 0.78g of lead iodide was produced, how many grams of lead nitrate was used? you may assume the reaction yield was 100% and an excess of potassium iodide was used.
given the product in grams.. how will i solve for the limiting reactant (in g), the excess reactant also is unknown..
Balanced would be: put a 2 infront of the reactant (KI) and a 2 infront of the product (KNO3). Yw.
But that is unbalanced chemical reaction
So balance it bro 🤜
thank you oh my god
potassium iodide is not aq, it is solid:)
THANKS
Tnx
Good 👍
This is a math problem more then a chemical reaction this video is bad you should feel bad for lying about the eucational value of this video anyone that used this as a reference🎉 I hope they got a f and could re take it if this was a source
It’s pronounced as Pb(NO3)twice
Ur trick is awful
It’s not always combining like that
It reacts according to the reactive series