Thermodynamics part 1: Molecular theory of gases | Physics | Khan Academy
ฝัง
- เผยแพร่เมื่อ 23 เม.ย. 2008
- Courses on Khan Academy are always 100% free. Start practicing-and saving your progress-now: www.khanacademy.org/science/p...
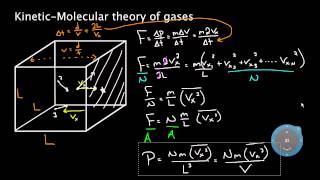
Intuition of how gases generate pressure in a container and why pressure x volume is proportional to the combined kinetic energy of the molecules in the volume. Created by Sal Khan.
Watch the next lesson: www.khanacademy.org/science/p...
Missed the previous lesson? www.khanacademy.org/science/p...
Physics on Khan Academy: Physics is the study of the basic principles that govern the physical world around us. We'll start by looking at motion itself. Then, we'll learn about forces, momentum, energy, and other concepts in lots of different physical situations. To get the most out of physics, you'll need a solid understanding of algebra and a basic understanding of trigonometry.
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s Physics channel: / channel
Subscribe to Khan Academy: th-cam.com/users/subscription_...



![แฟนแนวใด๋ - ยูริ โตเกียวมิวสิค [ SyncVersion ]](http://i.ytimg.com/vi/Fm8oDwos2uc/mqdefault.jpg)




u made me love physics so much !
U have a great ability of making students understand It's magical !
Honestly, the main reason I admire Sal is the fact that although at times he may stray from the topic, he always seems to return to his point.
Thanks Sal, needed this vid!!!
Best thing about this channel is that videos are downloadable
Very informative, getting ready for our Thermodynamics class. Hopefully i can keep up later on harder topics.
i just wanna say your doing a great job! im sure your helping so many people with your videos! thankyou!
This is one justification and the BEST use of youtube so far. Thanks for doing this Sal.
Brilliant. This really explains thermodynamics in an accessible and methodical manner. Many thanks.
Thank you so much! I'm pretty sure you saved my Physics and Calc grades. This makes more sense than anything I've seen in school.
Lol you probably already have a job and I'm graduating next year
@@oofaymanfahim Oh wow! I haven't thought about this in ages! It's funny, looking back. After a brief misadventure in accounting, I actually ended up getting into history education!
@@oofaymanfahim Best of luck getting through senior year! For me, that was the hardest point: I can't promise that adulthood is easy, but the freedom of choice is amazing!
@@agricolaterrae As a kid I always dreamed of being an adult but now that I'm a teen, I kinda want 50/50 from childhood and adults haha, thanks anyway ;)
@sokoke4152 Here's the good news: as an adult, you can still do all the stuff you liked as a kid! You'll probably always enjoy the stuff you enjoy now! And as a Ren Faire actor, I can vouch that there will always be plenty of people up for playing "let's pretend".
I have a thermodynamics test tomorrow for my college engineering class and this really helped (I'm a sophomore in High school) this was such a difficult unit
SCIENCE WOOOO WOOOOOOOOO
Hi cutie
Amazing, a lot of students would be very happy to have you as a professor. everyday 5 stars!!! i will recomend your videos to my mechanic profesor in order to get more simple xplanations in classroom.
you do a nice explaining this! please keep posting!!!
Words seems to be of very little significance to admire what you are doing for humanity. I can just say you are great!!
helps alot, especially how to visualize pressure
Would you mind made a class on Sound as well, I need help on that section.
A Teacher 4 whole world thank U Sir!!!
ty so much i couldnt belive it when i saw you have done this many videos for physics as well as chemistry
Thanks dude, it really helps out!
1) He is talking about an ideal gas in the absence of heat and work interactions, If you have heat or work PV is no longer a constant.
(For example external work due to compression causes temperature rise)
2) Real gases do in fact have a self-heating(or cooling)effect (called the Joule-Thomson effect) under compression due to friction, however this is usually ignored and is not predicted by Van der Waals' equation alone.
BEST TEACHER EVER!
great Sir....
very well explained thank you :)
very interesting thanks
ill show these vids to my kids from a very young age.
sal should re-do these very fundamental videos in HD cuz the old stuff is a bit fuzzy
Thanks, this was really helpful ! Greetings from Serbia :)
great great great..........thx alot keep it up
hats off to u bro..!!
nice vedio!! reduce the thickness of the text please!
Hey Sal, I think you should specify that Boyle's law (pV = constant) is only a special case of the ideal gas law (pV = nRT), that is, it only applies for ideal gases at constant temperature!
wonderful ... very good !
ur helping some ppl (meeeee) a lot khaan..keep up the awesomistical work :D
Touche hahahaha... it's important to solidify one's knowledge of the foundation before building upon it. This makes sure we've got the basics down. I'm thankful. One baby step closer to a degree!
good god viewing the menu... i'd love to have this guys breadth of knowledge.
you always prize intuition and I think that is good.
Does pressure increase as it goes on because entropy increases?
some 1 please give this guy a medal...
Would you mind talk about sounds as well?
For Aeronautical Engineering, which thermodynamics should I listen? Phsyics or Chemistry? I think I should listen phsycs but want to be sure.
I don't remember ever learning about thermodynamics until college.
Bloody hell we can make him the President of Education of the "World", Neatly Done!!
I love how youtube says the quality is 240p while the ACTUAL QUALITY of the video is much, much higher
nice vedio..
the best part
BAM BAM i dnt know BAM!!!
Basically, the molecules are bouncing off the walls, and pushing on the walls as they bounce. For example, if a dodgeball bounces off you, it pushes you, right? That's why dodgeballs can make you fall over. So basically, the gas molecules are all bouncing off the sides like dodgeballs or bumper cars, and the bounces push on the sides. But when they bounce, they don't lose momentum, the momentum just changes to the opposite direction: the particle goes at the same speed in the other direction.
I havn't watched any other videos, nor do I have any education in physics. Would the presure decrease over time because of the energy (or momentum) of the gas-particles being converted into heat (or something?) on the sides of the container? (Given that the container is not in a vacum and is able to give off the heat it has gained from being bumped into). This might be a really silly question, but I really don't know and would love if someone could enlighten me :)
he was talking about ideal gases which when it hit smthing it doesnt lose energy but they dont exist so yes the pressure will decrease by time but am not sure if am wrong tell me
Might be a dumb question but if you were to squeeze a balloon on all its sides completely would it pop?
No, a balloon pops because there is a pressure difference between the inside of the balloon and the outside, which stretches the rubber, to the point where it will tear.
Now, if you press _equally_ on all sides of the balloon, say put it in a pressure chamber, what do you think will happen? There will be less pressure difference between then inside and the outside of the balloon and the balloon will deflate a little, making it _less_ likely to burst.
You could keep going and going. And the balloon would get limper and limper and the rubber slacker and slacker, less strained and less likely to "pop", that is tear violently and let all the gas out in a rush.
Until the air inside the balloon took up almost no volume whatsoever, and as long as you get rid of the heat generated by the compression, there is no force whatever that would tend to cause the balloon to fail. Quite to the contrary, you are actually relieving the forces that could cause the balloon to fail.
+RzVids I know that this was 4 months ago, but MrGoatflakes is correct. You can test this theory by swimming with a beach ball. Push a beach ball to the bottom of a pool. The pressure will push evenly on all sides, and it would appear that the beach ball is deflating.
***** Since fluid doesn't have a definite shape, its pressure applies in all directions.
Well ... If understand your question and I believe that the it can not be practically applied bur theoretically if you applied the same amount of pressure on all points of the sides of a balloon it will be under the act of equal forces and it won't pop since the air has now where to escape and not enough force to Pop it up
Haven't you tried bursting a balloon? Try it if you haven't.
Thanks, man! Waht program are you using to draw? I would like to use ist here in Brazil!
i'm taking thermodynamics next quarter:)
I love this guy.
Medio raro esta este tema pero muy interesante a la vez!!!
Thanx a bundle! Now I can skip classes :)
perhaps it will get hotter due to other factors like increase in collisions and friction as the particles are in a smaller space, just a thought I'm not sure
Thank you sir
Thank u!!!!!
Actually you've made a valid point, but here's simpler explanation: liquids ARE in fact compressible! From my own experience with high pressure hydraulics for metal forming.
Example: A metal press has the main hydraulic cylinder with an 18" bore has been pressurized to 3000 psi. With 0.75% compression per 1000psi.; compression = 2,250 psi; total. If F=P*A; we have 127,170lbs total force!!!
Without the decomp valve, the hydraulic piping would burst!!
The result is a whopping 5,250psi !!!!
which software u are using to make such kind of ossum tutorial videos...????
7:30 actually blowing a balloon is generally easier as gets bigger.
Please do a Chemistry series Ap Chem. is so hard.
too good
as you increase pressure, heat increases aswell and so that heat rate must be measured too
Kahn used the symbol "K" for constant because it is a universal symbol for constant
For example: Coulomb's constant is
"K-sub-c"
Wonderful
I always thought that when you compress the gas, they get hot, that would contradict to PV being const. Don't ideal gases get hot when they are compressed? Does that happen only with real gas (van der Waals)?
khan I'm having a problem every few hours after eating I feel this pressure below my abdominal area and it just builds up and up until this brown stuff comes out...I have no Idea what's going on and I don't know what I should do..
you deserve a noble prize sir
Thanks niac video
thank you sir !!! can i giv u hi five !!! u make things simple
5/5 Thanks.
If the pressure in a gas is constant and in all directions, how come the molecules are described as going at different speeds - wouldn't they be uniform? Or are they accelerating after collisions? I personally believe that pressure in a gas is stored in the tension force between repulsive electrons. This means the particles could be stationary and the (if you like) elastic spring tension between forced together electrons provides a force in all directions which is effectively pressure at the container sides - the electrons attached to the atoms in the molecules, are resisting being contained.
this guy deserves the nobel peace prize
i feel like my college tuition should be forwarded to you
@hotcopone they didn't give him a medal, but they did give him 2 million for his work :) and to continue it of course
Also: The Deepest Point on Earth is the Mariana Trench. It is 11 kilometers deep wich results in a pressure of 1106 bar. That means the Density of water there is 1044 kg/m³. The Densitiy of Water at standard conditions is about 999.1 kg/m³.
WOOOO WOOOOOOOO WOOOO
Actually you can watch doctorate level lectures on youtube! That being said, Khan is the Man!
@mondaymorninbongtoke Watching videos is just the first step to learning. The next step is to actually apply what you've seen. In this case, you need to go and do some examples yourself, and solve the equations for different systems. You can easily fool yourself into thinking you know a subject just because you saw someone else do it.
is it heat that gives the molecules thier kinetic energy?
is it heat energy that causes gas molocules to move?
does the molecules never attain the state of rest?
I see no mention of "AP".
i passed in physics because of him hehe tnx a lot
Thank you ✌️🤙💪👏
Thank you very much . I really appreciate your work . I am studying thermodynamics in college of engineering but we didn't get this basic informations which make it easy to realize the difficult stuff we learn now . Thank you again ! Eng.basil from KSA
Hey, does anyone know why he wrote K for constant ?
"C" cannot be used as a variable as it denotes the specific heat hence "k"..
Can you teach at WSU?
ILOVEYOU KHAN
Thanks
thanx
@Pig560
The energy from the balloon burst is transferred to the air which causes the air to vibrate at a certain frequency. It's why any sounds occur.
I feel like I've a elder brother who knows everything.
he also said "exhales" instead of CO2
If ideal gases are volumeless point mass then how come area
@berniebay wouldn't little m mean micro?
That is what constant stands for.
Thank you, when I graduate, I will donate
how's that going?
@yamenhawit the chemistry playlist
@Pig560 maybe the air pressure relased moves the air monocules
@KaslarProductions You missed the fact he spelt "too" as "to" haha
Can someone help me ! how I can find density of gas using speed of gas's molecular !? (speed of molecular given from a m/s to b m/s)
Donno man,I'm bored
+1, i'm with him