Why p - orbital has a doublet in XPS Spectra - X-rays Photoelectrons Spectroscopy
ฝัง
- เผยแพร่เมื่อ 5 พ.ย. 2024
- Why p, d, and f orbitals have double peaks in XPS Spectra?
These double peaks are called Multiplet splitting or Doublet or spin-orbit splitting
One line answer is when there is unfilled shells containing *unpaired electrons*. For example, Mn²⁺= 1s²2s²2p⁶3s²3p⁶3d⁵4s² (where in 3d⁵, all five electrons are unpaired and with parallel spins, here we get doublet for 5d orbital like Mn 3d3/2 & Mn 3d5/2)
Let's explain it in detail
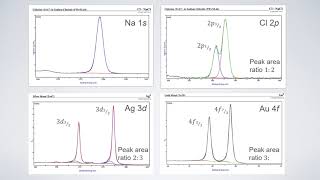
If the orbital angular momentum (𝑙) = 0, we get single XPS peaks like for s- orbitals such as 1S, 2S, 3S, 4S.....
If 𝑙 greater than 0, a doublet XPS peak, which means 𝑙 =1, p-orbitals, 𝑙 =2, d-orbitals, 𝑙 =3, f-orbitals
𝑥n𝑙j nomenclature for XPS doublet peaks like (Zn 2p1/2 & Zn 2p3/2), (Ag 3d3/2 & Ag3d5/2), (Pb 4f5/2 & Pb 4f7/2)
𝑥 - represents elements such as Co, Fe, Ti, Zn, Cu, Y, Mn,.....
n : principle quantum number, 1,2,3,4....
𝑙 : orbit angular momentum quantum number
j : total angular momentum quantum number; j = 𝑙 ± s (where s =±1/2 is spin angular momentum)
________________________________
For p-orbital:
For p-orbital, 𝑙 =1, n = 2, then
j = 𝑙 + s = 1+1/2 = 3/2 ( 2p3/2)
j = 𝑙 -s = 1-1/2 = 1/2 (2p1/2)
______________________________
For d-orbital:
For d-orbital, 𝑙 =2, n = 3, then
j = 𝑙 + s = 2+1/2 = 5/2 ( 3d5/2)
j = 𝑙 -s = 2-1/2 = 3/2 (3d3/2)
________________________________
For f-orbital:
For f-orbital, 𝑙 =3, n = 4, then
j = 𝑙 + s = 3+1/2 = 7/2 ( 4f7/2)
j = 𝑙 -s = 2-1/2 = 3/2 (4f5/2)
Source: Handbook of X-ray Photoelectron Spectroscopy by John F. Moulder