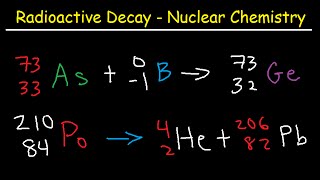
Half Life Chemistry Problems - Nuclear Radioactive Decay Calculations Practice Examples
ฝัง
- เผยแพร่เมื่อ 29 ก.ย. 2024
- This chemistry video tutorial shows explains how to solve common half life radioactive decay problems. It shows you a simple technique to find the final amount of the sample that remains and how to get that same answer using a formula / equation. This video contains plenty of examples and practice problems.
How To Balance Nuclear Equations:
• How To Balance Nuclear...
Alpha, Beta, & Gamma Decay:
• Alpha Decay, Beta Deca...
Half Life Chemistry Problems:
• Half Life Chemistry Pr...
Carbon-14 Dating Problems:
• Carbon 14 Dating Probl...
___________________________________
Nuclear Binding Energy & Mass Defect:
• Nuclear Binding Energy...
Nuclear Chemistry & Radioactive Decay:
• Nuclear Chemistry & Ra...
General Chemistry 2 Final Exam Review:
• General Chemistry 2 Re...
SAT Chemistry Subject Test Review:
• SAT Chemistry Subject ...
____________________________________
Coordinate Covalent Bond:
• What is a Coordinate C...
Complex Ions & Ligands:
• Complex Ions, Ligands,...
Naming Coordination Compounds:
• Naming Coordination Co...
Beer Lambert's Law:
• Beer Lambert's Law, Ab...
Crystal Field Theory:
• Crystal Field Theory
___________________________________
ACT Math Practice Test:
• ACT Math Test Prep
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tuto...








Final Exams and Video Playlists: www.video-tutor.net/
Full-Length Videos & Worksheets: www.patreon.com/MathScienceTutor/collections
Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
whoops. wrong half life
😭
Reallll😭
Hahaha
I came mid video to look at the comments. Yeah this is questionable but hey man, he's one hell of a genius teacher. I give him that!
Couldn't imagine how hard my life would be in chemistry just without you
Very nice video. Even a student of history graduate like understand the explanation. Thank you. Keep it up. ❤❤
THANK U THIS SAVED MY LIFE
This was helpfull! My proffesor (Dr. Isaac kleiner)
Made me have to calculate it with barney and gordon, But i didnt understand any off it, i heard he was talking about teleporters and stuff.
Forever grateful for your videos
Rise and shine, Dr. Freeman.
The most accurate equation or formula for number 1 sir 👇
= Ao (1/2)^HL
Where;
Ao is the original amount
1/2 is constant
HL is the Half-Lives
Solution using Ao (1/2)^HL
Given:
Ao = 200
HL = (32 ÷ 8) = 4
Solution:
= (200g)(1/2)^4
= (200g)(1/16)
= (200/16)
= 12.5
This guy is a machine
Thank you ❤️
Can you explain on how i van input the calculations on computer
Made by Gordon Freeman
Yeah half life 2 cool
HALF LIFE FAN LETS GOOOOO🧪🧪
You condensed a week long module into an 18-minute lesson for me, you are a life saver
My professor Dr. Freeman got mad when I used an easier method so I’m glad I watched this video. Thank you!
Dr. Freeman?
@@frankenwaifu8092 yeah I had him once, he was a mute and had an interpreter. Pretty cool dude
I feel his pain
😂😂😂😂😂job weldone👏👏
@@frankenwaifu8092 he is the main protagonist of the video game "Half Life
Not the Half Life I was looking for...
lol
and you clicked anyways?
Are you talking about half-life game?
you came looking for copper and you found gold
this is an underrated comment
شرحح ممتاازز !!thanks keep going 💪
Gordon doesn't need to hear all this, he's a highly trained professional!
The organic chem tutor is professional in teaching both math and science😄
Saruppu
ksmk
And finance, engineering and even how to run a TH-cam channel
guys how did you get the 2.7725887 answer
@@GabsARV he teaches finance too??
no... Half-Life is a Video Game
Instructions Unclear, accidentally caused a Resonance Cascade
My professor was furious at me when i used the easier solutions on the finals lul
bagakay ko same, I feel they so dumb in maths because they just think they are smart, but they don’t know other people can understand their complicated solution or not. I hate math course from university and college.
when you go to collage its the opposite lol
That's a bad teacher.
i once completed a exam without using any of the methods taught in lecture and my prof. handed the exam back graded, he had written on the top that he couldn't mark my work incorrect because i still got the answer right. But isn't that the whole point? to find the answer regardless of means (with the exception of cheating of course).
@@drwatsonater1381 I have had some exam questions specify the method they want to be used.. But most the time its simply find the answer snd show how you got it--irregardless of what method you used.
UH DID I DO SOMETHING WRONG THERES ALIENS TELEPORTING EVERYWHERE
This video is a life saver. I don’t understand my chemistry teacher’s explanations but this video really helped me out. Thanks!
A (1/2)^H=R
A= initial mass
H= number of half life
R= remaining mass
Yooo thanks
omgosh THANK YOU!
I might be wrong but isn’t it
N(t) = N0 * (1/2) ^ t/T
N(t) = Remaining Mass
N0= initial mass
t= days that have passed
T= half life
For the first example it would be:
N(t) = 200 * (1/2) ^ 32/8
N(t) = 12.5
k but how am I gonna remember that formula?? :(((
wait I have to correct myself: t= time that has passed
half life is my favorite game!
lol
cringe
What where are the head crabs and vortigaunts, where is Gordon Freeman at?
Sir your explanation is awesome and so vivid....Thanks a lot
If your calculator is fx-991ES plus like mine (or quite possibly could be applied to some) just press Alpha then x10^x button labeled as pie and you'll get the e.
Yooooo thx fam
😂😂Funny how someone with username cunningligus is out here saving lives
You forgot something you see there are 4 half life games not counting sources oposing force and blue shift however what goes in between the empty epace between two and episode 1 you get it
I feel my anxiety slowly going away 😭 Thank you, you are the God of stem 😘
Do you know who ate all the donuts?
1/10 not enough crowbars
So there are 2 equations that can be used and manipulated for all of these half-life problems:
A Final = A*e^(-kt) and k = (ln2)/half-life & algebra is used to move the stuff around.
Why did my instructor over complicate this so much?
The Desi Vegan my prof took two weeks to explain what you did in several sentences. Two weeks later and he had evertone confused and asleep....
what is the value of “e”? i don’t get it
@@unanimousd5403 in your T.I. Calculator just enter “e” to the power of “1”. It’s a constant.
As always, extremely helpful. Thank you!
Gordon doesn't need to hear all this, he's a highly trained professional!
Anyone here for half life’s but for physics class also?
Half life 3 confirmed
*insert certain game franchise jokes*
the problem is that hl3 is still not realised
Thank you so much! I am glad I found this video. I watched so many and they all were more confusing than helpful.
after u find k in the last example, couldnt u put it in for a diff. eq and solve to get 3.9999 = 4 instead of 5. Any way I could understand this principle in a better way?
perhaps im not understanding where the half life equation derives from?
@@kennedytaylor601 It comes from integrating the decay constant which can be expressed in a first-order differential equation. th-cam.com/video/dni8BrIOavw/w-d-xo.html
I was in science today and so many of us were confused! but i love our science teacher very polite and good at explaining but i’m just lost 😆 this helped very much as it went at my pace thank u!!
I like how you did both methods to solve these problems. Honestly, I actually find the equation to be easier to use because you get to find everything and just have it all in one setting to divide.
Same
This is a certified λ moment
GORDON FREEMAN!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!111111111111111111111111111111111111111111
For people that haven't learnt about the 'In and e^ thing' I've 'concluded' an equation from this video, hope this would help
(Initial amount) = (final amount)*(1/2^((exp time)/(half life)))
the exp time and half life should be in same unt, eg: both in days
this worked for me anyways, so idk if im wrong or not 😂
Nope! This is legit man! I your equation written in symbol form: N=N_0 * (1/2)^(t/h)
N= amount solving for
N_0= initial amount
t=time elapsed
h=half life
This works because whenever t is equal to the half life, we are raising the factor 1/2 to the first power which of course equals itself and that product times initial amount will equal half the initial amount which checks out with the whole idea of "half-life".
ex. problem in the video with iodine-131
N=200 * (1/2)^(t/8)
plug in the value for t = N=200 * (1/2)^(32/8)
= N=200 * (1/2)^4
=N=200 * 0.0625
=N=12.5
I'mma suggest everyone to use the formula then calculate by easy method.
Cuz exams don't allow easy methods.. but writing the formula will give you full marks
This is the best video I could have asked for. It answered my questions, and it was immensely helpful.
the way you solve problem is like you have magic in your hands, thank's sir I never knew that half life is this simple.
This channel has like every physics topic..Awesome!
so these equations can be applied no matter if the reaction is zero, first, or second order half life reactions?
This video stopped a mental breakdown 💪
2:44 WTH IS A LOG
HOW AM I LEARNING THIS IN THE GRADE IM IN?? THIS IS LIKE 10TH GRADE STUFD 7UP9FULI7CUL8YTRE JHVREZY34AHGIU,N
U shouldn’t have used this video to study cause I think it’s more advanced then the stuff ur learning
Btw there is an easier equation you can use that's really helpful
A(0.5)^t/x
its the same equation but not in two steps
This video was so amazing, I couldn't begin to express how much I appreciate the help!!
Could you explain what am I supposed to multiply for e like 200(e) ??
The value for e (the ln that is equal to 1) is 2.71828. On most calculators, like my phone's, you can just input e by itself and it will give you the full value.
@@JH-zo7rb i still don’t understand
hhhh im often wondering now when im listening to a tutorial/ lecture, why i'm not at home listening to organic chemistry tutor instead! i learn so much more from you! it's much closer to a one on one situation which is best
THANKS SO MUCH, i really needed to learn this for my CETs
Thank You, but how we can write the half life in terms of days and hours?
At 9:18..answer would be 26
Where the hell did e come from? what is e?
I wish you had been my professors for everything! I watch a different video everytime and I feel like I've learned more in this time then I had in 5 years
hello gordan
Infact this man is indeed a professor. I really like his teaching. How can a teacher make a one week or one month topic just turn into 18minutes?This man is very awesome.
Their waiting for you, in the test chamberrrrr
love your videos
i have better understanding of half-life thanks to you bro
do we need to subtract the two amounts to get the Af in the 4th question like in the 2nd question??
YOU ARE SINGLE-HANDEDLY GETTING ME THROUGH UNIVERSITY. THANK YOU.
When u get "K" you can also NOT round it off just yet
just type the full value so that ln2/0.1386294361 = 5
when i type ln(2/0.13863 the calc comes out with 2.669 ... confused >
Pls sir help me with this radioactivity calculation: A certain inhibitor was added to a chemical reaction, increasing the activation energy by 40% at 30°C. If the initial half life of the reaction is 55mins, determine the half life of the inhibited reaction(assuming the reaction is a first order reaction).
You are literally keeping my mental health afloat. Thank you
Pls Aaron I know you don’t know me but pls help me with my work it’s due tomorrow if u reply I’ll send you the questions lol
@@tragedy2722 Yeah dude, what do you need help with?
@@aaronius4444 bruhh 😭
I am so beyond confused and this just made me more confused. In conclusion I am going to go jump off the nearest cliff so I don't have to face my parents when they see my physics grade.
Will this solve the release date for half life 3?
do we need to convert years to days if we want to get the half life like in the first question
Radi0active Space not really, i think it depends on what unit of measurement (in time) is being asked for final answer
My teacher made this stuff seem hard but you make it seem easy. Thank a lot man!
So, lets suppose they put some radioactive Cesium in my son’s head to burn up a tumor. How do you find out, when there is no more left? We seem to play with half lives, and “Expect” them to decay linearly, but who is to say when each individual atom was created? Isn’t it just a crapshoot? If I isolate one atom of a radioactive element, how can I predict when I will have a “HALF ATOM” … or be gone?
No ones gonna see this but faster way for the first problem have 32 by itself times it by 1/8 you get 4 right. Then your gonna take 200 and times it by 1/2 to the 4th and thats how you get 12.5 faster
Have a good day (I have finals next week)
Thank you so much for this video, at least you can explain it correctly, verses my teacher. Keep on making more videos like these!
It's easy to understand half-life, because it's basically dealing with exponents of 2. But this a real help to calculate it, instead of getting a headache trying to do it myself.
Brilliant explanation - how about this. I had 370MBq of I-125 on 26 Mar. How much did I have on 16th Mar? Help please
youre legit so helpful i have a quiz tomorrow partially regarding half lives, I'll let yall know how it goes!!! THANK YOU
You are a fantastic tutor!! Thanks for taking the time to teach us. Your videos are so helpful.
I never went to class during this subject and this video alone taught me the entire concept, THANK YOU
ı understand nothing in the class but now ı know everything .thank you sir...
Half Life problems? Use an MP5 with an underbarrel launcher.
Can someone tell me what In2 on the TI 2s calculator and used for what? I’m trying to break it down and grasp the concept of conversions, etc
This is so helpful. You are such a life saver. Thank you so much
gr8 vids love them. but I think that the answer for question @ 8:50 is wrong, it should be 1.4 hours. I put it back in the equation and got the right answer. plus even when you look at it it takes 15 hours for the substance to go from 800g to 400g so how does it make sense that in 60 hours it went down only by 50g.
Thank you brother I am in class 8 but I couldn't understand the basics of half life taught by my teacher but you did taught me in minutes.... appreciate it man.
Activity is not measure in grams, your units are wrong! Grams will not decay, activity (Bq & Ci or dpm & dps) do decay.
Thanks very much! Just in time for my chemistry long exam :") More power!
Yeah, I have a crush on this guy! I always look for his videos because I like the way he explains things and clears up confusion! Keep 'em comin', sweetness!
Sameeee❤
Please im really stupid and trying real hard to finish my chosen degree 😂why did you choose 50 instead of going town to 25 ---> etc
Why does he sound like Murr from Impractical Jokers when he got his lips injected
Thank you, bro.
I'm studying Fire Safety Engineering at Ulster of University and this tutorial refreshes my A-level chemistry.
why can't we take the half life constant devide it by the days, than divide the square of the days with the initial quantity of of the element to derive the final quantity?
3:52
8:18
8:45
Please make it clear that there is no change in the mass of any decaying radioactive element. Just the isotope decays and therefore the number of disintegrations per second reduce by half in one half life, weight does not change at all. Iodine remains Iodine and sodium remains sodium.
This has been maybe my 6/7th time watching a video of yours in the past 5 days - very helpful!