Oxidizing Agents and Reducing Agents
ฝัง
- เผยแพร่เมื่อ 30 มิ.ย. 2024
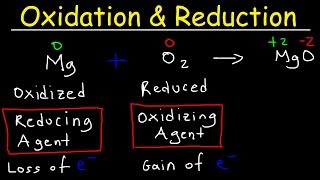
- This video tutorial shows you how to identify the oxidizing and reducing agent in a redox reaction. The first step in determining which species is the oxidizing agent is to start by finding which reactant was reduced. Likewise, the molecule or element that is was oxidized is also the reducing agent. That's how you can determine it. However, you need to calculate the oxidation numbers or states for every element in the chemical equation. Oxidation involves a loss of electrons and occurs when the oxidation number increases. Reduction involves a gain of electrons and occurs when the oxidation state decreases.
Stoichiometry Practice Test:
• How To Solve Stoichiom...
Solute, Solvent, & Solution:
• Solute, Solvent, & Sol...
Strong & Weak Electrolytes:
• Identifying Strong Ele...
Molarity Practice Problems:
• Molarity Practice Prob...
Ion Concentration In Solutions:
• Ion Concentration in S...
Dilution Problems:
• Dilution Problems, Che...
___________________________________
Types of Chemical Reactions:
• Types of Chemical Reac...
Solubility Rules:
• Solubility Rules
Predicting The Products of Reactions:
• Predicting The Product...
Activity Series of Metals:
• Activity Series of Met...
Will This Reaction Occur?
• Chemistry - Will The R...
Predicting Products of SR Reactions:
• Predicting Products of...
___________________________________
Double Replacement Reactions:
• Introduction to Double...
Net Ionic Equations:
• Precipitation Reaction...
Writing Chemical Equations From Words:
• How To Write Chemical ...
Solution Stoichiometry:
• Solution Stoichiometry...
Molarity & Dilution Problems:
• Molarity Dilution Prob...
Acid Base Neutralization Reactions:
• Acid Base Neutralizati...
____________________________________
Acid Base Titration Problems:
• Acid Base Titration Pr...
Mixture Problems:
• Mixture Problems
Calculating Oxidation Numbers:
• How To Calculate Oxida...
Oxidation and Reduction Reactions:
• Oxidation and Reductio...
Balancing Redox Reactions:
• Half Reaction Method, ...
Ideal Gas Law Problems:
• Ideal Gas Law Practice...
___________________________________
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections








Final Exams and Video Playlists: www.video-tutor.net/
Do you know you are out here saving people's lives with these tutorials? Thank you soooo much!
You guys are not from India we can study from TH-cam without even going to school because there are various channels...they teach full syllabus
Honestly, he’s saving lives better than going to school to those professors that can’t explain things well for students to understand.
@@ayanagarwaliitdelhi7662 got into iit or like just JEE Aspirants saying Future IITian
Where are you going from
The guy is good, very good and he has really helped where I couldn't understand.
After watching different videos on this topic after 2 hours...Alhamdulilah I finally found a video which cleared all my doubts😀thnk u so🤓 much!!!
The besttesttttttt lecture I have ever gotten on REDUCING AND OXIDISING AGENTS . Allhamdulillah now I have understood it. JazakAllah (thanks)
you are a great chemistry tutor i was trying to understand it for weeks and you help me in mints thank you soo much
COOL ENGINEERING
Thank you so much! This has definitely enhanced my knowledge on Oxidation-Reduction reactions!
I found comfortable to review back chemistry contents that I learned in high school by watching your video :)
Thank you so much! You just helped me in reducing a LOT of my homework and understanding the lesson!
You helped me in maths in 7th class, You helped me in math in 8th class, and now you are helping me in Chemistry in 9th class. Salute to you.
I wish you were my teacher, Love ❤ from Pakistan.
How much did you gain in chemistry in 9th?
Thanks a lot... I was unable to understand this topic at school even after 1-2 months but after watching the video all my doubts have been cleared... Thanks again... 😄
man thank u so much , my chem igcse is after 2 days and ur channel helped clearing all my doubts
Thanks for this video . This actually helped me in my exams . u made me understand it in minutes. THANKS 😊 😊
l love how you teach to people
+1 subs. increased .
now I can say that I am not that much bad in chemistry
your videos are really helpful, thank you so much (been away from classes for weeks due to contests and vids likethis really help me catch up)
wow, thank you soooooooooo much. words can't explain how grateful I am for this explanation.
This video is 6 years old but man is it a lifesaver. THANKYOU
Thanks man , this really helped me 😭😭🙌🏻🙌🏻
osm video thank you very much for this useful and needed video
You're the only one that has made any sense so far!
Seriously you're really saving us out here with the videos. Our good performance that we have in class we owe the credit to you. 🙏🙏🙏
even after such a long time, this guy is a life saver
i love all your videos! each topic has been so helpful for me and made me yell out "OHHHHHHHHH i get it!!!!"
Awesome, this video tutorial is very helpful. THANKS ALOT
World's best teacher........ Thank you
After watching it, I understand a lot. Thank you❤️
Well I am a tutor myself and found this explanation better than mine.... great job.thanks
you are naturally a good teacher --- for real or digitally, :)
The man is u the only chemistry tutor for me
You have the ability to teach things that I have to spend all class period trying to understand lol
this vid is a lifesaver!! thank u so so so much
very helpful. great quality video. thumbs up for sure.
God bless you! thank you for being sooooo great!!!
YOU EXPLAINED REALLY GOOD :)
It’s people like you who make my life easier
Yes this was driving me crazy thanks 🙏
Thank You sooo much...it made my concepts crystal clear...ty
4 years and nobody corrected his Hcl? It was supposed to be 2 Hcl so that the equation would be balanced but anyway other than that thanks so much for this video u saved another life here because my teacher didn’t teach redox he only touched on oxidation and reduction and expected us to know the rest and scolded us for not knowing how to do and not putting in effort to learn, or if not he just asks us to go home and learn ourselves instead of him teaching. So big thanks to you my life is saved :))
Edit: he keeps forgetting to add a 2 to balance lol but it’s ok
ah thanks for correcting then
I was just saved by this video. Thank You so much.
men you are really an intelligent men. chemistry. physics wowwwwwwwww u should be awarded for real!!!!!!!!!
U are better than my chemistry sir
That was an awesome explanation bro thankyou so much
You are a lifesaver!
finally i understood it! it was so difficult!
Thank you so much ur explanation was very helpful
Thank you so much for this best examples
you helped me sooo much thanks man🥰😊
Much informative. Thanks
After 2 days there is the test of this topic and tgis man help me alot thannnnkuuu verry much
AMAZING !!!! Thanks sooo much
This guy deserves the salary of like 20 teachers
You are a LEGEND!!!💖💖💖💖
this practice help me sooo much thank you
You're welcome
Thanks so much Master
Thanks for this video.
thank you so much!
Thanks for your videos
I love this...
2 years now I don't understand this topic
B
Thanks a lot amigo❤
This helped so much thank u
Please make a video on IODOMETRY
Thank You so Muchhhhhhhh
Thanks sir very helping
Thank u thank u thank u thank u💞💞💞💞💞💞💞
Thanks 👍
Thanq sir .....😭....it cleared my doubts 😌..cuz online classes make no sense....
Can your lecture explain way better than this ?
UGGHHHH THANK YOU SO MUCH
Sir for zn+hcl== h2+zncl2
Is balancing equation not nessasary
Awesome explanation 👌👌👌👌👌
Ok
THANK YOU
Your amazing
It was awesome helped me a lot
Thank you very much you solve my problem
Now i understand 👍
I love you man
How do I know which element I should find the oxidation number for, knowing that the question did not specify for me the element for which I should find the oxidation number?
For example, O2F2, what element should I find the oxidation number for?
Thanks...
Too good
thanks
What about chlorine in second equation ? 5:42
Is it oxidising agent or reducing agent ?
Great
Thank you
i appreciate it mannnnnnnnnnnnnnnnnn
thank you
Thanks
Sir can you please tell about 5clo3 + 3as2s3 + 9h20 = 5cl + 6h + 6h2as04 + 9s
Which one is what ??
thanks so much man
Ok
do you know how many times I've aced my exams because of your tutorials
Im confused, is bromine the oxidized or is it the sodium bromide as a whole? Gotta ask for my homework.
Why HCl is used in titration mixture?
K2Cr2O7+ 6KI+14HCl- I2 + 2CrCl3+8kCl
what about identifying reducing agent and oxiding agent in a reaction that is written as a decomposition? like product (compound) --> reactants hg + n2 or osomething like that
Alright let’s use the decomposition of water as an example. The equation would be 2H2O= H2 + O2. The oxidation number will be as followed +1 -1= 0 and 0. Hydrogen is getting reduced as it gained electrons. Oxygen is being oxidized as it lost electrons. Oxygen is being oxidized because of hydrogen so hydrogen is the oxidizing agent. Oxygen is the reducing agent since it is what cause hydrogen to be reduced.
Can you please help me out with this one 🙏🏽
State the property exhibited by nitrogen (iv) oxide in each of the following equations:
4Cu + 2NO2--4CuO + N2;
H20 + 2NO2--HNO3 + HNOz.
magician.
In the second reaction there 2hcl I think so
Becoz product contains cl2 and individual h2
Why do scientists make these things complicated
ty
I LOVE YOU.
Cool
Ans this :-
2Na + H2 --> 2NaH
Then who get reduced and who get oxidised ?
Na goes from 0 to +1 so it is oxidized
H goes from 0 to -1 so it is reduced
I think...
👌
Where does it get the +2 charge though. I'm so confused..
Because Mg is from Group 2, and it has 2 valence electrons. It will donate 2 electrons to become octet, therefore forming +2 charge. As for Br, it's fro Group 17 (or 7A) and therefore has 7 valence electrons. It will accept 1 electron to become octet, therefore forming -1 charge.
ChemSimplified ahh okay thank you very much!
@@Electric-Wind-Hook-Fist You're most welcome!
Thanks so much. Please, what is your full name?
does it matter in which state is each element?
I don't think so.
tysm