Ionic Solids, Molecular Solids, Metallic Solids, Network Covalent Solids, & Atomic Solids
ฝัง
- เผยแพร่เมื่อ 8 ก.พ. 2025
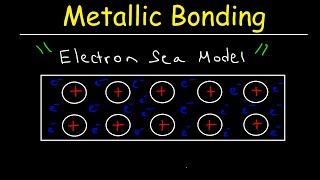
- This chemistry video tutorial provides a basic introduction into solids. It explains how to classify a solid as ionic solids, molecular solids or atomic solids. There are 3 different types of atomic solids that you need to be familiary with - metallic solids, Group 8A solids, and network covalent solids. Ionic solids are typically made up of metals and nonmetals. Ionic solids contain ions with positive and negative charges. Molecular solids are composed of molecules and have very low melting points. Ionic solids typically have high melting points. Metallic solids are composed of metals with varying melting points. Metallic solids conduct heat and electricity very well. They are ductile and malleable. Group 8A solids which are basically the noble gases have extremely low melting points. Finally, the network atomic solids or network covalent solids have a very high melting point which typically varies with pressure.
Chapter 10 - Video Lessons:
www.video-tuto...
________________________________
Lewis Structures - Mega Review:
• Lewis Structures, Intr...
Sigma and Pi Bonding:
• Sigma and Pi Bonds Exp...
Hybridization of Atomic Orbitals:
• Hybridization of Atomi...
Molecular Orbital Theory:
• Molecular Orbital Theo...
Dipole Dipole Forces of Attraction:
• Dipole Dipole Forces o...
_______________________________
Hydrogen Bonding:
• Hydrogen Bonds In Wate...
London Dispersion Forces:
• London Dispersion Forc...
Ion Dipole Forces:
• Ion Dipole Forces & Io...
Bragg's Equation For X-Ray Diffraction:
• Bragg's Equation For X...
Molecular & Network Covalent Solids:
• Ionic Solids, Molecula...
_______________________________
Metallic Bonding:
• Metallic Bonding and t...
Metal Alloys:
• Metal Alloys, Substitu...
Diamond Vs Graphite:
• Structure of Diamond a...
Semiconductors:
• Semiconductors, Insula...
Unit Cell Chemistry:
• Unit Cell Chemistry ...
_________________________________
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tuto...








Chapter 10 - Video Lessons: www.video-tutor.net/liquids-and-solids.html
Can you please make a video where you explain the differences in intermolecular forces🙏
This guy explains stuff a million times better than my professor. Absolutely goated channel
THIS GUY LITERALLY SAVED MY LIFE IN GRADE 11, AND IS SAVING IT AGAIN IN GRADE 12. GOD BLESS YOU DUDE!!!!!
Now you might be about to graduate university or just have a job 🤔.Wonder how these knowledge from 6 years ago have influenced your life
this dude saved my life in ap physics and now hes saving my life in ap chem
it seems we're both in the same boat
Eject dude
idk what id do without this guy like hes literally teaching me physics chem and calculus 😭😭
I swear this guy is better than all of my teachers for any class, you are awesome!
I learned this concept better because of you than my chemistry teacher taught me. Thank you so much !
Not that I hate my chemistry prof., but I love you so much better. You literally helped me understand this concept and how to know what type of solid it is based on the way they're written Thanks a bunches!!!
Sammeee
I couldnt seem to understand it at alll
this guy is the reason I have an A in chem
Dude thank you for these practice questions. I am over as your level two member at Patreon, btw.
Professor Organic Chemistry Tutor, thank you for an exceptional Introduction into Solids in AP/General Chemistry. Solids are used in all types of materials on planet earth. Solids that have a high/low melting point and those that conduct electricity are an example of real life problems on planet earth. Professor Organic Chemistry Tutor, there is a typing error in problem 3. There should be a "C" instead of "D", "D", that is, you have, "A", "B", "D, "D", "E". Please correct the typing error in the video.
I think I’m in love with you ? 😩
:')
IKRRR😫😫😫🤞
Lmao 😂😂🤣
I need you to come with me to the police station
real
its quite easy to understand now.. thanks
I just love this guy..thanks loads
Literally the best teacher ever
Thank you so much for explaining these. I found it very helpful.
Wish Kenyan chemistry teachers were like this.its amazing
thanks for still coming in clutch in 2021
Thanks for your time ❤
You're a great teacher
Thank you, Mr Gonzales.
You misspelled "soft." What you meant to say was that molecular solids are S.A.W.F.T..
The last option of diamond was about Chemistry is full of exceptions 😂 thnk you for the lecture
what's the equation for crystalline solids?
Gallium is below Aluminum not Indium in case anyone cares
Why this video has such less views...??
Can someone explain to me why AlN is a covalent network solid if it has ionic bonds?
I thought you would make a video about the types of voids and more
So, If I want to know that the thing that looks like diamond is indeed a diamond can I hold by one end of the thing and the other end attach to an electroshock.
would that work ?
it depends if that other thing that looks like a diamond is conductor of electricity
it depends if that other thing that looks like a diamond is conductor of electricity
How can the Ionics have higher melting points if they are in the negatives, would they not be lower?
That’s not a negative sign it’s a hyphen
Are ionic solids more brittle than molecular solids?
NH4Cl
Ionic solid?
awesome
Tysm
Brass is which type of solid?
it's an alloy so it is metallic substance since alloys are mixtures
@@Bereket2D ok
801 °C for Nacl ? no?
No
Ilike very much I am from ethio
gallium is the one that is below aluminum not indium
P4 is which type of solid?
Agcl3 is which type solid?
ionic
I think ionic
Iodine is which type of solid
covalent network solid
molecular?
Its a molecular solid both solid iodine and sulphur
It will have london forces
Molecular (? Maybe
legend
Here for modules y'all
4:42
Gallium, Germanium, Arsenic...
I love you
Dude u made a mistake it's positive sign in-front of (MgO)
Oxygen can form a double bond with Magnesium
FACE REVEAL
whaaatt😳
I hate my chemistry teacher
SAME DUDE
voice is too low. please increase the voice in other video