Complaint Handling in Compliance with FDA and ISO Regulations
ฝัง
- เผยแพร่เมื่อ 16 พ.ค. 2024
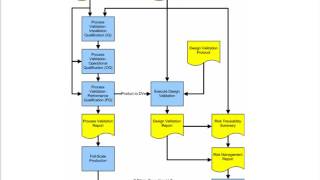
- Negative customer feedback about a medical device's performance or safety is a strong indicator of whether a firm's manufacturing process is in control. This feedback is therefore subject to many requirements in both the QSR and ISO 13485. Failure to follow up on complaints about medical devices is among the most frequently cited observations on FDA-483s.
This Video will show the best way to document customer feedback, what constitutes a complaint, and what do with "non-complaint" feedback. Also contained will be a suggested method on including complaint trending into your firm's CAPA program. Additionally, the application of risk management to a complaint handling system will be reviewed, and a specific risk management system explained.
This video presents an overview of FDA's requirements for approval/marketing of a Biosimilar (Generic Biologic) product. The webinar covers the Biosimilar product testing requirements (clinical and nonclinical) and the Biosimilar approval pathway.
The video also reviews the Regulatory/Scientific/Quality principles involved along with the 3 FDA Biosimilar guideline documents. In addition, the FDA Stepwise Approach and the FDA Totality of the Evidence concepts will be discuss.
For More Information Contact -
Organization: NetZealous BDA GlobalCompliancePanel
Website: www.globalcompliancepanel.com/
Email: support@globalcompliancepanel.com
Help us caption & translate this video!
amara.org/v/JGYn/








Whoever who is speaking thank you so much for your help dude
I was part of this "Complaint Handling" course in the year 2012. It was Fabulous course.
Hope another course will make it available online with new updates.
Hello Jenelle Cotter, Thanks for your words, and just subscribe our channel and you will get an update for new courses. We will Keep updating courses.
Hi
I need help in one scenario please help
Great learning. Well explained. Best video I have seen.
Hello Thanks for Words....
@@globalcompliancepane Is there a more current video on this topic?
Great session