Regulatory Documents Explained - DHF, DMR, DHR and TF
ฝัง
- เผยแพร่เมื่อ 12 ก.ค. 2016
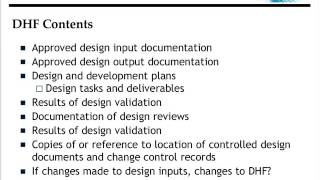
- The FDA QSR and the Medical Device Directive specify certain records that should be included in your organization's quality system - Design History File (DHF), Device Master Record (DMR), Device History Record (DHR), and Technical File (TF).
For More Information Contact -
Organization: NetZealous BDA GlobalCompliancePanel
Website: www.globalcompliancepanel.com/
Email: support@globalcompliancepanel.com
Help us caption & translate this video!
amara.org/v/Q7i1/