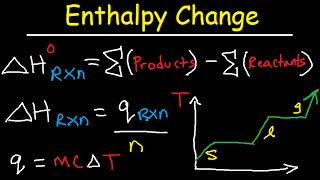
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
ฝัง
- เผยแพร่เมื่อ 28 ธ.ค. 2024
- This chemistry video tutorial explains how to calculate the enthalpy change of a reaction using the enthalpy of formations found in the appendix section of your textbook. it also explains how to calculate the heat of combustion or enthalpy of combustion of a substance using the sum of products minus reactants formula. This video contains a few practice problems for you to work on.
Thermochemistry - Free Formula Sheet:
bit.ly/3TP4U4u
Chemistry 1 Final Exam Review:
• General Chemistry 1 Re...
______________________________
First Law of Thermodynamics:
• First Law of Thermodyn...
Thermochemistry Equations:
• Thermochemistry Equati...
Internal Energy, Heat, and Work:
• Internal Energy, Heat,...
Thermochemical Equations:
• Thermochemical Equations
Specific Vs Molar Heat Capacity:
• What Is The Difference...
________________________________
Basic Calorimetry Problems:
• How To Solve Basic Cal...
Final Temperature Calorimetry Problems:
• Final Temperature Calo...
Latent Heat of Fusion & Vaporization:
• Latent Heat of Fusion ...
Coffee Cup Calorimeter:
• Coffee Cup Calorimeter...
More Calorimeter Problems:
• Bomb Calorimeter vs Co...
__________________________________
Specific Heat Capacity Problems:
• Specific Heat Capacity...
Hess Law Problems:
• Hess's Law Problems & ...
More Hess Law Problems:
• Hess Law Chemistry Pro...
Enthalpy of Formation & Heat Combustion:
• Enthalpy of Formation ...
Enthalpy Practice Problems:
• Enthalpy Change of Rea...
__________________________________
Speed of Light, Frequency, & Wavelength:
• Speed of Light, Freque...
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections








Thermochemistry - Free Formula Sheet: bit.ly/3TP4U4u
Final Exams and Video Playlists: www.video-tutor.net/
Chemistry 1 Final Exam Review: th-cam.com/video/5yw1YH7YA7c/w-d-xo.html
I was stuck for hours on combining equations of formation to make a regular equation and the first 10 seconds of your video enlightened me. God bless you
Omg same
literally same
Same here
Bruv if this is the level of physical chem people have in the west every asian would top the test.... its way hard over here!
@@whoKnowsWho27 is it though?
When you have to learn Chemistry else where because you're under lock down and your teacher won't answer your emails ....
btw. you just taught me something is less than 20 mins, which i've been trying to understand for the past 6 days so thanks
This man has been carrying my grades from high school into college lolol
i can relate veeery much
SAME
@Cody Bowie dude that guy is a scammer and a bot
Heck yeah HAHAHAA
Did someone say...... *illegal activity* ?
I stopped listening during Chemistry lessons cause I literally don't understand my teacher's teaching..I just can't wait to come home and listen to your explanation on this topic...Thank you...I'm now the highest scorer in Term 1 ..Just scored (98/100)...Everyone was shocked..I'm just sad that I discovered your channel late...I'm now in my last year of high school...Should have started watching you from Junior level 😢😢....
Best luck all of you for exam tomorrow 🤣🤣
😂😂
Exam today 😂😂😂
My exam is today too
Test tomorrow 😒
Thx I’m totally fcked
I cannot thank you enough for your videos!!! I went and bought some chemistry books but they hardly give any practice problems! You make it much easier and approach problems from different angles, thank you!!!!
No one teaches this as simply as you do. Thank you
It's a great pleasure meeting a wonderful teacher make difficult topic more clearer. I now understand better now.
Put it up and God will bless you
Thank you. You are a blessing when it comes to straight to the point lectures. In fact, better than Khan Academy, and teaches way better than my chemistry professor. Just have to search the textbook for a certain topic and then search it on TH-cam
Thank god this video exists....
Thank him...but then he IS god
@@degraj418 Woah chill
I learn here more than in my class
Professor Organic Chemistry Tutor, thank you for a step by step explanation of How to Calculate the Enthalpy Change of a reaction using Enthalpy of Formations in AP/General Chemistry. The problem selection for this material are excellent. Every problem in this video also reviews the concept of balancing Chemical Equations in Modern Chemistry. This is an error free video/lecture on TH-cam TV with the Organic Chemistry Tutor.
Thank you so much! All of your videos have been a huge help for my first year of pharmacy school. Cheers!
woah I didn't know a 1/2 coefficient was allowed
The coefficient of the product has to be 1 unless stated otherwise, so a 1/2 is required for the reactants
@Megan choo To form water you need one oxygen and 2 hydrogen atoms
Do you like black people?
The reason being is that the atoms of oxygen always exist in pairs; that’s why there’s a subscript of 2 in front of O. All diatomic elements are this way (H2, N2, F2, etc). If diatomic elements are always in pairs, how do we get just one atom of it? You would have to multiply the subscript 2 with something to give you one. The only way you would get one atom is by multiplying it by 1/2. Does this make sense? The 1/2 doesn’t mean half of one atom; it is half of 2 which we know is 1. It is another way of writing one atom of Oxygen in this equation.
@@yabombo8145 what does this have to do with anything?
So glad I waited for you to graduate before beginning this journey. Much love bro. South Florida love
Organic Chemistry Tutor The GOAT
Thank you so much ur carrying my grades from 9th grade to 11th grade thank youu so much
The enthalpy changes are measured kJ instead of kJ/mol since you multiply everything by moles. Love all your videos btw.
Thanks
Better than my chemistry professor by a mile
3:31 for making this more understandable we can say whenever 1mole compound is form then enthalpy of reaction becomes eq to enthalpy of formation and when 2 or 3 etc moles of compounds are form then we have to divide the moles from enthalpy of reaction to get enthalpy of formation.
That does it. Imma start building a shrine for this guy.
1:31 you have to put graphite because its the main form of carbon (solid) and thanks alot great video
THIS VIDEO IS GOLD 🥺🥺
9:19 why is the result in kJ per mol? It looks like it should be for the entire reaction.
this guy is a gem
Great video, I'm wondering though why you don't use reactants minus products in the combustion reaction of c2h5oh, since it's a combustion reaction that includes oxygen. I've learned that combustion reactions are always exothermic, so in this case it brings a negative sign which means exothermic, so I'm good with that, but HOW do I know when to use products minus reactants or reactants minus products? Do I just switch signs if I get a positive value when I know it's a combustion reaction? Little confused here. Thankful for answers.
ikr shouldve dlne Reactants - Products in that one its the rule isn't it
Is there a balanced synthesis equation for ethanol, or does ethanol only form through combustion?
Never mind, I found it.
Thank you for this! Not many textbooks explain this concept properly.
Lecture Liya b h ya bas comment sy hi Kam chla lia😂
@@tahaprince6978 hi
who's studying for finals with me 🥴
Lmao right here
Right here 🥴
Good luck to both of y’all
@@hussnainshakeel2372 had mine. This video saved me on the Enthalpy questions haha. Thanks. Hope your finals go/went well too.
I am studying for the finals that I will have one year from now xd, this Thermochemistry stuff is one hell of a challenge I am studying on a text book, I also have a few other "note book" things that should help with the text book, I have 50 practice problems, I have like 30 tabs opened, I will watch the mit open course ware lectures on thermochemistry and then I will be done. I don't know why I told you this, but after writing it down I feel a little better :')
We have an exam in thermo chemistry today. thank you for making this video. Wish me luck!
Ronn Carl Faminialan how’d u go?
@@milindbordia he probably died
Your tutorials have saved me once again, thank you
You should be my professor!
A PRODUCTIVE VIDEO....KUDOS TO THE TEAM
You are the best man🙏king
Around 9:30
I have a similar question where butane is burned to produce CO2 and H2O. My professor insists that the value for liquid water is to be used in the formula instead of the water vapor one. What I don't understand is why does the value of water vapor not apply to this situation. I don't remember cooking on the butane gas stove with liquid water coming out of it.
Any explanation that can convince me?
Yeah there is explanation
Just don’t think too much
Thank you so much for this!
Thanks alot bro, it make's easy to understand thermochem
Why am I watching this? It's 2 minutes to midnight and I don't even go to school anymore!
Well explain and easy to understand
Could you explain why dH(rxn) is -1396kJ PER MOL at play time 9:25. I thought dH(rxn) is -1396kJ for 4mol-NH3 and 7mol-O2.
For anyone else who wonders, I believe he made a mistake writing /mol and it should just be kJ. If you follow the units of each number on your head, the moles cancel.
You explain things so well. Are you a TA or prof or something?
Absolutely Brilliant 👏 👌 Thank you so much 😊
Please strongly explain to me what is the relationship that connects the heat of combustion with the heat of the formation
Thank youuuu this is so helpful
Thank you very much man you're AMAZING
welcome
Thank you so much sr.You save me from failing the exam.😞
You simply the best
I have an exam tomorrow and I still don’t understand Heat of Combustion 😀 wish me luck before I head to my certain doom!
Thank u so much good job keep going.
Another great video. Thank you!
Learned a lot
He's the best
you are the best dude!
Yato gang?✌️
I wish I could know which grade this lesson is for, bc I am taking it in high school second grade in Saudi Arabia, and thanks man for this video ❤❤
Thanks you soo much brooo made my life easier
Thank you for such a great video :)
Beautiful video. Nice job
Thankyou so much for the awesome videos!
how can this piece of flesh can teach that amazing !!
thanku sir.....awesome lecturing
you've saved me for tomorrow
my goal is to fulfill my purpouse in life to the same degree you have fulfilled yours
thank you. thank you. god bless you.
THANK YOU SO MUCH! CAN you make for heat of combustion please...
Thank you. This is great!
ohh man you are awesome
my goat 🐐
Good one sir.
How do i know if the product is gas liquid or solid?
16:15 isn't supposed to be +552 not negative?
-856 = -1408 - 6x right
552 = -6x
divide both sides by -6
x = -92 kj/mol
It’s second period and my test is fifth period, wish me luck 🥴
How did your test go?
Good luck y'all for finals 😅
What about calculating the enthalpy change of formation if given the enthalpy change of combustion?
Enthalpy change of combustion is enthalpy change of reaction
And to solve for that is
∆Hproducts - ∆H reactants
you can use algebra and finish up
@@hectorokereke9268 thank you
Do you only put elements on the reactants side?
2:01 Anyone know why there is a 2 after O? Why can't he just write O so that he does not have to put 1/2 in front
oxygen is a diatomic molecule, each molecule of oxygen comes as 2 oxygen atoms. it is naturally found this way so you still have to write it that way. its odd and confusing but thats science for you.
This guy sounds like Mark Whalberg
yess thats what i thought too
What is the balanced synthesis equation for ethanol?
Never mind, I found it.
will the carbon dioxide and water formation enthalpy always be the same for problems or does it rely on the textbook
life saver !
thankyou so much.........
Thanks 🎉
This is so easy.
333K views. 3 years ago
Nice ❤️
Question:
Enthalpy of formation of H2O is 0 or -286? What do I miss?
Thank you!
This is probably a bit late but it’s -286 because H2O (L) is not a “standard state”, it’s created from the formation of H2(g) and O2(g). So the H2 and O2 are 0 not the H2O
Thank you.
Its 8:36 and my test is at 9:20 wish me luck 🥲
Helpful,
May I ask how is it possible if we undergo combustion raction while still staying at standard condition (1 atm, 298 K)?
Some tough numericals needed#IITJEE ....PLZ.....ON THERMOCHEMISTRY
Why wouldn't your units "mole" cancel out when solving for the heat of reaction? I think it should.
If I am not given the KJ/mol of the compound how and I supposed to figure out the heat formation of a compound?
Not sure if this is a dumb question or not but if I have an equation with with no pure element that is in its standard state that equals 0, then how do I find the zero to make my math work? How do I determine what the pure element is if there is none already set up in my equation?
thanks for this video
The goat
yo the organic chem tutor r u teaching A levels chem?
Thank you🇳🇬🇳🇬🇳🇬
people how did he get -552 at 16:15. i tried divide the 856/-1406 but kept getting 0.60. can someone explain to me if i misunderstood pleeeeaaase
Why would you divide? You have to substrate.
-856= -1408-6x
6x= -1408+856
6x= -552
x= -552/6 = -92 kJ/mol
Why is the enthalpy change of the reaction is equal to the enthalpy change of the copustion of 1 mole
Great!!
Whats the point of listening in class for 45 minutes and not understanding when I can listen to your 15 minute videos and understand everything.