Is NaOH +HCl Exothermic or Endothermic?
ฝัง
- เผยแพร่เมื่อ 29 ก.ย. 2020
- In this video we'll determine whether the reaction of NaOH and HCl is exothermic or endothermic. Since the addition of NaOH + HCl = NaCl + H2O we can identify it as neutralization reaction. Neutralization reactions are exothermic.
We can also look up the change in enthalpy (ΔH). Since ΔH is negative for this reaction, heat will be given off to the surroundings and we have an exothermic reaction.
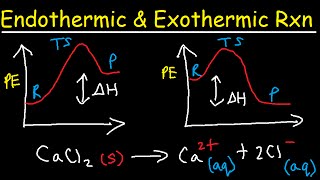
-Exothermic-
Heat Released (to surroundings)
Temperature (of Surroundings) Increases
- ΔH (negative enthalpy)
Products are at a lower energy.
Examples : Burning Paper, Neutralization Reactions, Respiration, Freezing,
CaCl2 + H2O
-Endothermic-
Heat Absorbed (from surroundings)
Temperature (of Surroundings) Decreases
+ ΔH (positive enthalpy)
Products are at a higher energy.
Examples: Thermal Decomposition, Photosynthesis, Melting, NH4Cl + H2O
-- Other useful videos for NaCl + NaOH = NaCl + H2O--
How to Balance: • How to Balance NaOH + ...
How to Determine the Type of Reaction: • Type of Reaction for N...
How to Balance the Net Ionic Equation: • How to Write the Net I...








I tried to think about why it’s exothermic and came up with the following explanation...
Everything in this reaction is a spectator ion except H+ and OH-. So we see that two “charges” are coming together to form a bond. Since forming a bond releases energy, this reaction is exothermic.
Thank you! I needed this for my homework today
@@salihak.1691 glad I could help!
Does this mean that the solution gets colder?
Exothermic means is gives off heat. So that gets warmer.