Zaitsev's rule | Substitution and elimination reactions | Organic chemistry | Khan Academy
ฝัง
- เผยแพร่เมื่อ 16 ก.ย. 2010
- Courses on Khan Academy are always 100% free. Start practicing-and saving your progress-now: www.khanacademy.org/science/o...
Zaitsev's Rule for E2 and E1 reactions. Created by Sal Khan.
Watch the next lesson: www.khanacademy.org/science/o...
Missed the previous lesson? www.khanacademy.org/science/o...
Organic Chemistry on Khan Academy: Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Let's make some beautiful organic molecules!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s Organic Chemistry channel: / channel
Subscribe to Khan Academy: th-cam.com/users/subscription_...



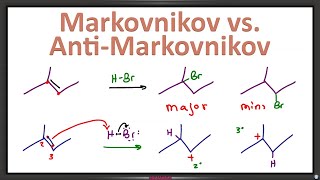





Fun fact:
Markovnikov comes markov, which is the russian for carrot
Zaitsev comes from zaets, which is the russian for rabbit
teachers really need to start using color-coded markers like this. it's so much easier to follow!
Hello where are you now ?
You are so much better than my professor and my book at explaining this. Thanks so much!
I love khan academy, thanks for being a calm and thorough resource for ochem, I wish professors could be this relaxed and patient with explanations
Khan is the reason I am doing so well in college.
Thank you for your clear explanation!! It quite easy to understanding the concept by using different colour.
you are the best chemistry teacher in the world
You just taught me the entire chapter, your awesome!
kinda missed out a lot about saytzeff's rule regarding the stability of the carbocation formed during the reaction.
Thank you so much Sal :) This helped so much!
i love the way you teach and the colors you use.. you are a talented chemistry master ^_^ thank you a million times
i love this T_T THANK YOU!!! Organic chemistry would be so much fun if it is always taught with this black background, pretty round handwriting, neon colourful writing, and CLEAR explanations!! Exam tomorrow so this is such a relief that I Finally understand the Zaitsev rule :P
are you still there?
thankyou so much!
If I remember correctly from Orgo if you have a bulky base such as tertbutanol then the lesser product will be more commonly observed due to the base having a harder time to reach the inside proton so it goes for the primary carbon instead?
Khan, Are you god? or just god in disguise?? you have delivered me from eternal failure, I actually love chemistry now, thanks to you!> you must be god!
in the final product with the R' and R'' why would it be cis? wouldn't trans have less steric clash and be more stable?
how do we know which base is strong and which is weak ???
@kourosh89
Than we should say it in that sense. The way that he said it does not depict the same meaning.
your website liiks awesome, much more beautiful and organized
hmm what if the carbon which is going to donate h was attached to 3 carbons? would it simply take from the other side?
I just do not think 2 mistakes within a video is acceptable. Especially when you guys are the respectable tutor online
This dude sounds drunk at 0.5 speed
broooooo im dying hahaha
Yes
Thankyou!
Gracias.
thanks !! :)
You said in an E2 reaction, the weak base will take an H from the beta-carbon; in your example with 2-chloropentane it makes sense and works well. HOWEVER what if you have 2 halides and a strong base for an E2 reaction (from a workbook)? Here's the question:
2,3,-dibromobutane goes through double dehydrohalogenation w/ 3NaNH2, NH3 in H2O solvent to give butene.
Do the e- from NH2 take H's from the 2 alpha-carbons OR the 2 beta-carbons followed by hydrogen shifts, to form the triple bond?
Sal Khan is definitely colorblind!!!
look at 2:11
Essential for organic chemistry
For condensed version start @ 6:20.
👍👍👍👍 awesome
Doesn't it work because Primary carbocations are unstable compared to tertiary/secondary ones?
Wouldn't the two carbon hydrogen be a more frequent target because of the electron density too? I mean, if there's only two hydrogens, and if carbon is more electronegative than hydrogen, well it now only has two hydrogens to steal from, so it would steal a little more from each hydrogen than the carbon with three hydrogens would. Wouldn't this make these hydrogens more positively charged and thus more liable to be grabbed off by the negatively charged methanol?
ok sure but whyd you do this as an e2 reaction then...methoxide anion in methanol solution ?????? how is that e2.
plus the more stable isomer is the trans one (usually), not the cis one he drew
AGREE !
Khan Academy on video E1 E2 Sn1 Sn2 reactions example 1, you mentioned that for the reaction to be E2 or Sn2 the solvent must be Aprotic (no free proton), however in the case of CH3OH, the oxygen has the ability to lose its H making it protic solvent. So i'm confused now. Can you or somebody explain??
The h with ch3oh isn't acidic,if we remove the h,the remaining base ch3o- will be highly destabilised hence ch3oh has no tendency to lose its h.( ch3o- is a very strong base so you can also say that the conjugate acid of a very strong base will be a very weak acid).Well that is what i think is the reason.
I cant see your pointer
i laughed when he say where he has more real estate. Hahaha
Write it down there. It's free real estate
Hyper Khanjugation
when i need to study for ochem exams, i have a date with you
I think I'm finally getting it! 😂
You're god.
#36
What program is he using?
paint and freedraw3
doesn't oxygen have a valence of 6?
I have a chemistry test tomorrow, if i pass it i will invite you to launch 😂😅
Hassan Ghost *lunch
Mess
it's pronounced "sir-jevs" rule
"I am a stone...."
in not land
It's saytzeff
Its actually pronounced as 'saytzeff'. The name of the scientist was Alexander Zaitsev
MY MILKSHAKES!!
I have been watching a lot of your video of chemistry and organic chemistry. It seems like you read about the these from the textbook but you haven't really taken any of these classes. In this video, you have got the definition of the arrow wrong. The arrow means the transfer of a "pair" of electrons and not just one. Also this is a E2 reaction and the hydrogen of the bottom should be taken. The leaving group should not be on the same side of the hydrogen get taken.
nevermind
rich get richer and poor get poorer cool.
Firstname Lastname the irony is that this was developed in soviet russia
chemistry sucks ;0