Describe the instrumentation of Fluorometry? | Analytical Chemistry
ฝัง
- เผยแพร่เมื่อ 4 ต.ค. 2024
- Many compounds absorb ultraviolet or visible light and undergo an electronic transition from low electronic energy levels to high electronic, energy levels. The instant re-emission of the absorbed
energy is called Fluorescence.
That instrument with the help of which we can measure the fluorescence a phenomenon is known as Fluorimeter.
That instrument with the help of which we can measure the fluorescence phenomenon is known as Fluorimeter.
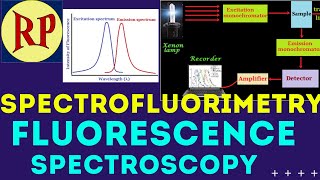
In this case beam of light travels in a single optical path is most commonly used light from mercury vapor lamp is allowed to pass through a primary filter. It allows only UV light to pass through it and absorb the visible light then UV light is incident on the sample holder which is also known as Cuvette. Here the UV light is absorbed by the sample molecules. The light which is coming out from sample holder contains fluorescent light and may contain original UV light. The emergent light from sample holder is then analyzed at the 90o from an incident in order to avoid in the mixing of reflected or transmitted light with the emergent light. This emergent (fluorescent) light is then allowed to pass through a secondary filter. This filter will absorb any of UV light. Now the emergent light is only fluorescent light and is then incident at photocathode Digital display which gives us the intensity of fluorescent light.
First, we have to standardize (Calibrate) the instrument by taking blank (solvent) in the cuvette. With the blank, we adjust the dial reading to zero with the help of calibration knob. Now the instrument is standardized and then we perform the experiment with the sample solution. For a sample solution, if we known dials readings then we can have a quantitative analysis by plotting a graph of F (intensity of fluoresce) Vs C.
Analytical Reasoning
• Solve Problems Related...
English Grammar
• Making Sense With Tens...
Interview Skills
• Questions You May Be A...
Managerial Economics
• MCQ #1 of Managerial E...
Royalty Free Stock Footage
• Flowers - Royalty free...
Chemical Thermodynamics - Physical Chemistry
• State and Explain firs...
Ionic Equilibria - Physical Chemistry
• Derive expression for ...
Electrochemistry - Physical Chemistry
• What are different typ...
Solid State - Physical Chemistry
• Explain the following ...
Gaseous State - Physical Chemistry
• Postulates of Kinetic ...
Colloidal States - Physical Chemistry
• What is Colloidal Solu...
Stereochemistry - Organic Chemistry
• Explain Configuration ...
Nanomaterials - Engineering Chemistry
• Compare top down with ...
Water and Its Treatment - Engineering Chemistry
• Explain why hard water...
Electrochemistry - Engineering Chemistry
• Distinguish between me...
Environmental Studies
• MCQ on Environmental S...
Optics - Applied Physics
• What are cartesian sig...
For Details Visit
cepekmedia.co.nf
cepek.hol.es/
edmerls.66Ghz.com/
edmerls.tk/



![The Wall Song ร้องข้ามกำแพง | EP.213 | กรีน อัษฎาพร | 3 ต.ค. 67 [1/5]](http://i.ytimg.com/vi/3KrlLbCsadI/mqdefault.jpg)




Awesome video. the vocal content being written in description box has helped a lot to prepare notes .
Why add unnecessary background music dude?
Where did you get the visible light from again mentioned about 2:08 after the excitation filter have only transmitted the UV light?
the sample after absorbing UV light, emits Visible light but some of the UV light can be scattered from the sample so the light coming from sample contains both UV and visible light which needs to be filtered for only fluorescent light which is Visible light only.
It's called stoke's effect. it's the principle of spectroflurimetery where substance absorbs a light of lower wavlength(uv) and emits it at higher wavelength i.e. visible light
Thanks
Awesome ♥️
please what is the name of the music in the background?
Thank you!!!!!!!
plz add video relates to Photofluorometer
Florimetre and flourescent spectroscopy same?
S
Yes
By which compound copper oxide Nanoparticles combines to produce fluorescent complex??
Hi
Bhaiya thoda kam awaz Mai baat kiya karo.. 🤩👍
Bhai jara slowly batao na.. Likhne ke liye time nahi milta
You can find written notes from cepekmedia.co.nf/
Bhai screen ke beech Mai dandi aate hai usko dabake dekh PAUSE ho jayega... 💐
play back option is available for speed adjustment.
👏👏👏