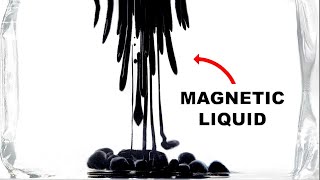
Attempting to make a ferrofluid
ฝัง
- เผยแพร่เมื่อ 15 ต.ค. 2018
- In this video, I'll be attempting to make ferrofluid from the ferric chloride and ferrous sulfate that I made in a previous video. At first, I thought that this project would be really straightforward, but it turned out to be much more difficult than I thought.
I am going to be working more on this project, and I hope that soon I'll have an easy method to make it from relatively easy to get chemicals and supplies.
References:
• Coated nanoparticle photo source: goo.gl/CtTsxU
• Making Iron (II) sulfate: • Recreating one of the ...
• Making Iron (III) chloride: • Making Prussian Blue
-------------------------------------------------------------------------------
Merch - nilered.tv/store
-------------------------------------------------------------------------------
■ NileRed is now available on Nebula! go.nebula.tv/nilered
(when signing up with this link, a portion of your membership directly supports the channel)
Join the community:
Patreon - / nilered
Discord - / discord
NileRed Newsletter - nile.red/home#newsletter
You can also find me here:
Facebook - / nilered2
Instagram - / nile.red
Twitter - / nilered2
Nile talks about lab safety: • Chemistry is dangerous.
Music in credits (Walker by SORRYSINES): / walker - วิทยาศาสตร์และเทคโนโลยี








"Cheap things like printer toner"
Lol
Lol
Lol
Lol
lol
Lisa Simpson's hair is ferrofluid and her skull is a magnet
edit: i dont remember making this comment and i hate it just as much as you do
LOL
oh no
get that idea away from me
R/cursed comments
stop that
Just wanted to say, the FerroFluid wrapping down the screw was one of the most relaxing things to watch.
Damn, catalyst must have studied this video for years.
TH-cam algorithms are sure awesome
This video is sponsored by EA 😂
@@John4Real 😭😭
Lmaooooo
Was the first thing I thought od
2:47 pause gentely jiggle your screen and it will appear to move
Oh god that’s messing with my mind
How
DUDE NO
lol
Ah shit how did this happend
"I definitely added way too much kerosene"
**Ten seconds later**
"So I'm just slowly adding more kerosene"
**0.2 seconds after**
"I think I used too much kerosene"
kerosene
I think it would be interesting to see if you can figure out the commercial solvent. I bet they don't use kerosene and that might make a big difference.
Christopher Walkensen who kerosene’s
Sometimes science isn't an exact science...
@@CharlesBosse polyethylene glycol perhaps?
This channel really opened my eyes to how fun chemistry can be, if only schools did these kind of experiments from time to time
But there legally required to be boring
These can get dangerous really quickly, do you really trust highschoolers to do this lol. In Uni chem you get to do a lot of cool stuff though with some pretty high tech equipment.
So that’s where my missing canisters went
I knew someone would comment something like this 😂
Me: *needs to sleep*
My brain: NO watch the CHEMISTRY MAN
Its literally 2am here haha
Me at 4 in the morning
So you and your brain are different beings?
@@triptomoon9870 its called a joke
Chemistry man... He awaits...
This venom trailer looks awesome
Holy fuck
Wear a magnet suit and pour this on yourself
Venomize that shit
Stop it lol
exactly what I thought of lmao
@@jasonfalzarano7888 yaaaas
I know, right!
“Pierce through the mundane”
Who else got this recommend to them after season 15 of apex?
Me
I don't think enough people tell you how professional and high quality your content is
Nice name. Where's that come from ?
Yup
second that!
:)
@@NileRed you are also undetrated, you deserve waaay waay more but ye you got an extra sub from me😁 and nice video man💯🔥
"Unsuccessful" experiments are just as important as the other kind. They should also be published.
Nile blue???
Nileblue
@@J.PC.Designs The kind that unqualifiedly prove the hypothesis tested
Catalyst saw this shit and started plotting💀
0:48 the forbidden blackberry fruit snack
If you're watching on mobile, pause the video at 2:39 and slightly jiggle your phone back and forth.
What the heck. That’s pretty cool, my dude
I thought I saw Ender man for a second.
Strange wonder why that happens
I have no idea how you found that 😂
I saw that too !! Is it the black contrast that provoke this effect ? That's a surprising thing.
"H2O is water" is the smartest thing i can say in chemical
@aud_io Liquid Gas
Dihydrogen monoxide
Hey I also know H2SO4 and NaCl 👆
CO2
liquid ice
OMG! THIS PERSON HAS LEAKED CATALYST SKILL 4 YEARS AGO! WHAT AN INSIDER
I think this is why I love your channel, you're willing to show everything, even your failures or mistakes. Most science channels only ever seem to show a perfect product, but you offer an insight into the problem solving process whenever something goes wrong, and explain your thought process when figuring out what went wrong, why, and how to fix it. The problem solving is just as much a part of science as the "cool stuff" is.
Some tips from someone who have made a lot of magnetite nanoparticles in the past.
1. How pure the iron salts are can have a big impact on nucleation and there for particle size. The time the solutions spend in air will affect oxidation and hydrate complex formation. I always used chloride of both Fe II and III, never tried sulphate
2. Temperature during precipitation is important, colder is usually better based on experience and transmission electron microscopy (TEM) analysis.
3. Do not dry the particles fully, try and make solvent exchange, this will prevent aggregation. For example: water - acetone - toluene - kerosene (perhaps there are better routes, I have not done this myself, also I am not sure what is most compatible with kerosene)
4. Ultra-sonic treatment is often mandatory for good dispersion.
Good luck :)
I made the experience, that Fe-NP from a hot wall reactor a much easier to handle and redisperse. You could put these particles in some sunflower oil and get a fairly stable ferrofluid. Chemical synthesized Fe-NP are very hard to handle, mostly because of the ligands. Not a fan of this method to produce NP, espacially for a purpose like this.
My next attempt will use an ultrasonic bath and an overhead mixer missing it super quickly. I will also use a nitrogen atmosphere to limit the oxidation.
Why is it that some people tell me that heat is absolutely necessary but others say that cold is usually better?
@@NileRed Im not an expert but on all the synthesis on nanoparticles that ive done most papers call for extremely dilute solutions in order to prevent aggregation, maybe if you drop down the concentration aggregation shouldn't be that much of an issue
This depends on the synthesis you're pulling off. Using a hot solution to inject the reuducing agent, you'll get a monodisperse phase with the NPs being pretty homogenous in size (called the Hot-Injection-Method). You'll need a dillute solution and relatively strong coulomb or sterical inhibition. Using cold solutions can lead to higher concentrated suspensions, I'd guess, and can get along with less good inhibiton. Your particle growth and size are going to be more heterogeneous, though.
I did it both ways once and I have to say that the temperature can get quite high if your inhibitor is good. Usually, sterical inhibition is good for long-term storage and more reliable, but Citrate was able to stabilise our Au-NP solution at temperatures around 80 °C by coulomb repulsion.
Me, who knows absolutely nothing and just watching this thread progress
NileRed: "It looks like magic"
Me: "I think that is Venom"
Even I thought it looked like venom
10:35 his gloves stuck at under the magnet xD
No amount of training can prepare you for using the right beaker size ❤️
Lol
That sounded wrong... was it meant to?
I need to get my ears checked. You said “hard drives and speakers” and I heard “hard drugs and sneakers” and was intrigued
Could just be your brain.
Must be all the hard drives you've been taking.
I heard hard drugs or speakers
I heard drive ways and speakers for some reason. XD Brains are weird
Or you need to check your speakers.
i don't know anything about chemistry, and i never understand what u do. but it's always so incredibly interesting just seeing everything react with each other and you doing stuff and trying to explain it to my tiny brain. well done dude.
Same thing m8
“Mom, can we make ferrofluid?”
“We have ferrofluid at home.”
The ferrofluid at home: 1:28
Try evaporating the kerosene away until it spikes. Also, the brown color may not be from contamination but rather the particle size. Nano particles have different optical properties from macro scale particles.
Safety Lucas true, like nano gold is red
Pretty sure its from the iron, its such a charachteristic color. Not to mention that its a mix of 2 colors, so it would have to be two very specific sizes of particles. Highly unlikely.
nano gold particles can easily reach small sizes, that's why they are prime examples for varied colors. Iron oxides? Pretty tough.
Xuan Bach Lai you can make nano ferrite down below 5nm
Xuan Bach Lai it takes other kinds of processes then the standard process he's using though.. But it possible
Clicked because I thought it was a 3D render (tutorial), stayed for science
**insert creative reply**
@@daneilgame123 *insert creative reply to your creative reply*
@@thelocalnecromancer1224 **insert really uncreative reply to your creative reply to a creative reply**
@@eyemoisturizer *insert really creative reply to your uncreative reply to a creative reply to a creative reply*
@@vergil2 *insert a mildly creative response to your really creative reply to their uncreative reply to a creative reply to a creative reply*
Pierce their hearts! And their feet
-somebody who is from Peak Myths or something
Catalyst definitely watched this video
lmao
Definitely
Actually admits early on and confirms this is an attempt and failed as to prevent any possible clickbait. You my friend are better then a large majority of TH-camrs , also very entertaining!
In high school I was really enthusiastic for Chemistry and pretty good at it too, a chemistry degree was my second choice.
Watching your videos (not this one in particular) makes me recall why I was so enthusiastic and makes me want to do some chemistry again! :D
This comment made me happy for some reason.
This comment made me happy for some reason.
And what was your first choice ?
this definitely only showed up on my feed because the new apex character
6:16 ‘forbidden coffee’ 😁
ALWAYS PUBLISH YOUR FAILURES! Very happy see this! 😊
Im glad my parents published me now, thanks!
Ichbims Derepep this is such a bruh moment
This was by far the best episode of Full Metal Alchemist
XD
@Chairman of Toes Don't throw your stupidity here.
Sarcastic Studios r/murderedbywords
This is alphonse giving us a tutorial
Full metal alchemist when the alchemists discover full metal idk i didnt watch the show
Ferrofluid: *exists*
apex legends fans: catalyst
This is so interesting, can’t wait to see further progress
I don't mean to sound like a know-it-all because I have no clue but do you think the stir bar you have may have had an adverse reaction as far as the magnetic movement
My thoughts exactly: "You're trying to make the magnetite particles as small as possible, but you're putting a magnet into them and pulling them together when you're trying to coat them"
Yeah same as what I thought (though I barely know more about chemistry than your average layman). I kept waiting for him to mention something about it but...
I'd have to disagree. I made a good quality sample that formed spikes while using the stir bar. The ratio of concentration of Fe2+ ions to Fe3+ ions is an absolutely critical component to making proper particles and is documented in literature. The ratio of oleic acid used also plays a noticeable role in sample quality if I remember correctly. The only issue I had with the sample was that i left it overnight in the ammonia solution it was made in and returned to brown gunk like that in the morning. So the only sample I have left was from my first attempt which also just made a small bulge like in the video, but stayed in suspension it was of lower wuality because I did not get the ratio of Fe2+ to Fe3+ ions down as close to perfect as it needed to be, it is very sensitive to deviation from that ratio.
Also as a side note, I also recall having that hydrophobic clumping thing occur. I don't remember how exactly was resolved but i think it was by dripping a bit of HCl in to lower the ph again. And mrhomescientist had a great video on how to make ferrofluid, which Is pretty much the procedure I used just slightly changed up some things to have higher precision. th-cam.com/video/LlQw9dfexBQ/w-d-xo.html
Good thing about it though is that it will discriminate by mostly affecting larger particles with bigger total magnetic moment(larger volume since it looks like single domain will be prelevant). For a given magnetic moment there should be maximal particle size stable on its own in solution. IMO stuff stuck to stirbar can be discarded outright. I might post later if I get not lazy enough to calculate limits imposed by the magnets. If somebody wants to do it separately it is just using Boltmans law to calculate when equilibrium concentration on magnet surface (proportional to e^(-m H)/kT) is enough so that distance between particles is enough so that energy of dipole interaction between particles is around kT.
Nothing is better than a nice calm educative NileRed video at the end of the day
dopplers effect :- th-cam.com/video/nUIuDsVezmM/w-d-xo.html
dopplers effect :- th-cam.com/video/nUIuDsVezmM/w-d-xo.html
16:07 looks like him pouring coffee ☕ 🤣
Nevermind pausing it. Just screenshot it. Truly mindboggling. What type of wizardry is this.?? NileRed you sir are dabbling in black magic.
Never worry about showing failed attempts , tbh its more interesting and people would prob learn more
I also like showing the failures. They are usually a better lesson than the sucesses
@@NileRed nile weezy can u please do a video on making white phosphorous from red one
9:10 "Mr Stark i don't feel so good" times 100.
C mamut
Lol
Bruh I just got an ad :'(
100? The ad said 3000.
THE BLIPP
Catalyst's favorite TH-cam video
4:24 certified breaking bad moment
EHEHAHEHAHAHEHAHAHEHE
@@mazidul4902hehehehhehehehhehehhehehehhehehhehehhehehehhehehehehhehhehehehehehhehehehehheheheheheheheheehhehehehehehehhehehehehehhehehhehehehhehehehehehehehhehehhehehehehhehehehhehehehhehehhehehhehehehhehhehehehehhehhehehehhehehehehhehehhehhehehehehehhehehehehhehehhehhehehehehehhehehehehehhehehhehehehehhehehehhheehhehhehehehehhehehhehehehehehhehehehehhehehehhhehehehehhehehehehhehhe........................................
2:39 if you’re watching on your phone shake it lightly while staring at the powder
I saw this on my own and made a comment damn its cool
Ya man its right its pretty cool
That's cool😂👍🏽
Oh no my eyes their broken
It also work when light watch night when not can see border and shake
1:28 "Cheap things like printer toner" made me laugh uncontrollably
Innit
I guess the new Apex Legends season must’ve brought me here
It's awesome to see someone that actually trys
Hey, have you got my mail about this topic?
-Add the iron solution (1.5M) to the ammonia, not the otherway around. The fallout should be blacker than yours.
-don´t pull out the magnetite straight from the solution, it will clump up. Instead add your oleic acid, about 5ml per 30ml of iron solution and bring it up to 80°C. ( I use Fe(II)Cl und Fe(III)Cl, I never tried the sulfates.)
I never got the magnetite crash out by itself, I usally disolve it in petroleum, seperate the phases and crash it out with acetone from the petroleum. Everything left in the water is thrown away, because these particles aren´t coated enough.
After crashing them out with acetone and a magnet, I redisolve it in petroleum (or n-Hexan/ n-Heptane). After this you should see small spiked when placing a magnet benath it. Then you have to supersatured the solution by letting it sit in the sun, or a another soft heat source for 2 weeks. It will thicken up and some bigger particles will fall out of solution.
13:15 this guy actualy gave me the idea of using acetone.
17:19 the color of your particles is way of, check the quality of your starting reagents.
19:10 spiking only acours when the solution is highly concentrated.
nice
Really helpful
you should've start by saying ''smart person to smart person''
Him: it really isn’t that complicated
Me: *aRe yOu SuRe AbOuT ThAt?!*
Yes
I know i'm getting wooshed, but this comment isn't funny.
AldobernalTV Bernal getting wooooshed means that they don’t get a joke, not if they don’t think it’s funny
@@bebanana181 indeed - unfortunately that won't stop people from misusing it.
@@aldobernaltvbernal8745 r/wooOooOOoOSh jk I understand
daamn apex science moment
i listen to this dude and learn nothing but still enjoy it
From what I know about pigment science, your problem isn't "filtering" particles that are too big or not coated. Your problem in the criticality of milling your particles. Milling after mixing with oleic acid should both reduce particle size, and force the lipids to bind. Unfortunately, milling is also a seriously tedious process, and often introduces contaminants in the form of grit. But it is a noble task, and I think it may be worth that effort.
Yes. It sounds to me like the problem is proper dispersion and wetting of the solid particles. In the paint industry sand mills are often used, where the pigment particles get coated and ground with a proper wetting agent and/or resin, and then stay properly dispersed once thinned with other paint ingredients. After 10 to 15 minutes of milling with external water cooling, the sand is filtered out through a strong metal mesh screen while the mill is still turning, forcing the thick liquid through mesh. Not sure if a small amount of fine sand particles would prevent the iron particles from working properly as a ferrofluid though, it would just have to be tested. I don't think Nile would have easy access to this type of a setup though.
It sounds like that would destroy the coating. I mean it will certainly "break most particles in half", wouldn't it?
What it does is divide agglomerates of particles and surrounds each particle with some of the dispersant so the particles won't re-agglomerate and will stay dispersed in the fluid medium, which is the end-game desired. They are using oleic acid in this case, but other longer chain molecules could possibly be used and might even do a better job at dispersing. A certain amount of trial and error is often involved in finding the best dispersant for each type of solid particles you are trying to disperse.
@@moronicpest I imagined them being crystals. Ok :)
dopplers effect :- th-cam.com/video/nUIuDsVezmM/w-d-xo.html
Well, now we know why it's so expensive...
What a gr8 video!!!!!!
I loved the where he said:
"IT IS NILEREDDING TIME!!!!!"
Foiler fluid made me chill its so satisfying
At around 4:24 my dad walked in my room and asked “Is ThAt CrYstAl MeTH?!?!”
Then everyone clapped
Cat with human ears lmaoooo
I have a theory you are from new Mexico and that your dad is a meth cooker who ran away to Alaska
bruh-
@@marciaosullivan3200 This isn't even a good thing to lie about! Why would you lie about something like this, it's not even something that couldn't possibly happen. People most of the time lie about their job or whatever (example: "I was in the FBI once!") I hate it when replies like these are on comments that have a big chance of being true, it's good to doubt things sometimes but this is like "Hey! There's a 20% chance this story isn't true, time to type out "Then everyone clapped" because everything that has a small chance of being untrue _IS_ untrue!" Thanks for coming to my ted-talk! Have a good day/night!
Hey I know this isn't the type of thing you normally do, but can you look into doing a video on alkalides? They're salts where the anion is a negatively charged alkali metal. I first heard of them a few days ago and looking at the procedure for their synthesis, it seems really interesting, though fairly expensive. Plus I haven't found any videos on them anywhere on TH-cam, so it could be a neat thing to show off
Can you share the synthesis? I'd bet it needs an inert atmosphere, negatively charged alkali metals sounds like it just wants to be with water.
I also want to see this. :D
@@stefangadshijew1682 na- in water??!
I looked it up, its not stable in normal atmosphere and nile does not seem to have the proper equipment to deal with this kind of synthesis (Schlenk line or glove box)
this seems very unlikely, but electrides are interesting as well and more viable
@@ouroya I honestly thought electrides would be harder to make. Also yeah he probably doesn't have what he needs to do it but it's still an interesting thing to think about, so I figured it was worth bringing up
This showed up in my recommended feed right after I watched the Apex Season 15 trailer
I find it sooo soothing to watch 😁🥰
Youre video got featured on a Daily dose of Internet Video! I'm so happy.
What one?
Which one?
Who one?
Why one?
How one?
somehow i always make my way here when im baked.
Baked?
@@thelocalnecromancer1224 it means high
@@MarvelousButter no
@@thelocalnecromancer1224 wdym no
@@MarvelousButter no, no
Never gets old watching.
This is one of those things you'll see and be like: "That makes me feel funny"
Hallo Nile. The reason for your Brown colour is that you have a lot of rust in it. I had the same problem at university. After you produce your nanopartikel, you need to was them with water and enthanol. Then dry them and do the step with oil acid at the next Day. Then you can take the particels and suspend them in Olive oil. If you want, you can take a soap Solution and put the suspension inside. Then you can get a very nice ferrofluid :)
English isn't your first language, is it?
@@UnbipentiumM he's most probably Indian or German
@@mannyheffley9551 surely not indian but how does it matter ?
@@nilaksh007 his grammar is why.
@@blue.1, I'm a native english speaker and despite his misspelling and grammar, I could still understand what he was saying. Why's it a problem?
Some things to consider:
1) In general nanomaterial synthesis, the surfactants are included in the reaction mixture during nanoparticle formation to stabilize the particles and restrict their growth.
2) For a ferrofluid to work you must produce superparamagnetic magnetite, which means the particles size should be on the order of 20 nm or less.
3) Though aqueous precipitation methods are common, you have less control over particle size. One way around this is to first form the organometallic complexes (Fe-oleates) and then proceed with the nanoparticle formation (though these are typically high temperature reactions), the particles will form while maintaining a complete oleate coverage on each particle and remain very small and highly crystalline which will improve the yield of superparamagnetic particles with a high magnetic susceptibility.
3) The color changes from black-brown-black during your purifications are (imo) most likely due to aggregation in the various solvents as the overall diameter of the aggregate changes so will the color and the rate of separation. Note that oxidation of the magnetite over time (especially in aqueous solvents) will lighten the material to a lighter brown over time and iron species at the surface will dissolve into solution which can eventually desorb your surfactant and destabilize the material.
4) You may wish to degass your aqueous reaction mixture to reduce oxidation.
As others pointed out:
1) Sonication! it's something I could not live without when it comes to nanochemistry (can get a jewelry cleaner pretty cheap and should work just fine for most uses).
2) >98% purity iron salt hydrates are pretty cheap, try and get some.
3) NaOH can be used if you don't need the ammonia-oleate reaction.
4) In many cases, the faster nucleation takes place, the smaller and more uniform your particles should be.
5) Magnetic stir bars will not adversely affect your reaction as long as you particles remain small and stable enough (as you correctly state they will not separate because their solubility is greater than the magnetic attraction force), however, if your reaction mixture is too large, mixing can become a problem and stir bars typically will not compete with a good overhead stirrer.
Anyways, great to see you covering some nanochemistry and a room temp benchtop method as well! Good luck!
This popped up on my TH-cam recommended.... I like it and it’s very interesting. I’m subscribing
17:26 slowest toilet ever bruh
Hey NileRed, love your Videos - you'll probably not read this because I'm late to the party but: If you're worried that the Magnetite oxidizes too much while stirring, you could try to do that process under a non-reactive heavy gas. You'll need to prevent excessive spillage of the gas but you'd rule out oxidation.
No one:
Absolutely no one:
Not a single person:
My TH-cam recommendations, at 3 am: Attemptimg To Make Ferrofluid
Almost 3:00 am
literally 3 am rn
Yo its 4:31 for me
Love how this is now trending cuz of apex
Take a shot every time he says kerosene
Nile red and elemental maker posted videos within minutes of each-other? BEST DAY EVER!!!
Lol he got his glove stuck under the magnet and ripped 😂😂😂
10:35
*Catalyst has entered the chat*
I'd love to see a part 2 if it's still a project your working on
He already made a follow up to this, a few videos later.
Can’t wait for the next batch!!
Challenge: making ammonia or urea from meat or lentils
Syilver Wors this isn’t entirely related to your comment but it’s inspired by it, just wanted to say if you don’t already watch Cody’sLab you should!
He made urea with his own pee lmao
Man, you would be the best science teacher ever.
This reminds me of the movie Splinter with how it moves and looks, it's pretty neat!
Me:watching the video
My 6 year old cousin :iS ThAt MagNeT JuiCe?
Your cousin is right
3:44
Forbidden orange juice
21:32 , Nile - "I'll try to edit it in a reasonable amount of time". Me looking at the video's time "Sure"
I love how he always sounds confused or unknowingly
Hi, I'm an automation engineer from India.
Many years ago, I have seen colloidal iron produced by electrolysis in a chemical factory. They were using a electrodes of iron to electrolyse caustic soda! I'm not a chemist & that time it didn't strike me about ferro-fluids. But their product was like bright grey-suspended in paraffin-oil.
Is there something relevant for you in my observations?
I looked at ur channel and... you don’t look like an automation engineer.
@@heaven4012 Youre stupid
@@heaven4012 What job do you have? Whatever it is, you're lying because you don't have videos that make it seem like you have a job! That's the logic you had in that comment, but i hope you've changed since then since it's a comment that was made a year ago.
@@heaven4012 *ok lemme just make people know my job is this is TH-cam and hopefully they think that it's my job.*
@@heaven4012 what... was an automation engineer supposed to look like?
Hey, I did research on magnetite nanoparticles. In my opinion, the brown thing you get is most likely iron (iii) oxide due to the rapid oxidation under air. Perhaps you can try to do the reaction under an inert atmosphere. Otherwise, play with the ratio, and because iron (ii) will be oxidized, you can try to add more iron (ii). Another suggestion that may be useful is to use charged surfactant as tetramethylammonium hydroxide, and do the coating process under sonicator rather than stirring. It helps to break down the aggregates better than stirring. Good luck!
You are correct. Magnetite is very unstable under oxygen and oxidizes to maghemite, which has its brown colour. But maghemite nano particles still are superparamagnetic and have almost the same saturation magnetization as magnetite.
I was the type of chemistry student that would probably cause an explosion with toenail clippings and water. Yup. I said it. But I love chemistry with a passion and I am really glad I can watch the process from afar. If I was in your lab I'd probably look at something and cause an explosion. Thanks for uploading. Any word on creating Spidey shot yet???
This gut took my chemistry grades from f to a+.Thanks man
"I washed it with some kerosene and maybe a bit of acid."
Are you not sure whether you washed it with acid?
YES TH-cam IS BACK UP!
I lied but it's back up now
Yeah, I also had a brief outage around 10. Most obnoxious.
This was so awesome, I'm going to go ahead and watch something Ive always wondered about... The Myths of Iodine.
I saw the thumbnail and thought it was the worlds most terrifying butt plug.
it makes me feel better about trying things knowing that even the people i look up to make mistakes sometimes
2:28 is it me or the powder is jiggling
nah i see it to
2:39 this powder is creating an illusion that it's slightly moving but it's not (pause the video)
🤯🤯🤯🤯
Yooooooo
Catalyst?
great video, I'd love to see what you'll come up with after further experimenting with it. I am thinking about doing something similar soon