Types of Chemical Reactions
ฝัง
- เผยแพร่เมื่อ 10 มิ.ย. 2017
- This chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or combination reactions, decomposition reactions, single replacement reactions, combustion reactions, double replacement reactions, precipitation reactions, acid base neutralization reactions, redox reactions, and gas evolution reactions. This video contains plenty of examples and practice problems.
Stoichiometry Practice Test:
• How To Solve Stoichiom...
Solute, Solvent, & Solution:
• Solute, Solvent, & Sol...
Strong & Weak Electrolytes:
• Identifying Strong Ele...
Molarity Practice Problems:
• Molarity Practice Prob...
Ion Concentration In Solutions:
• Ion Concentration in S...
Dilution Problems:
• Dilution Problems, Che...
___________________________________
Types of Chemical Reactions:
• Types of Chemical Reac...
Solubility Rules:
• Solubility Rules
Predicting The Products of Reactions:
• Predicting The Product...
Activity Series of Metals:
• Activity Series of Met...
Will This Reaction Occur?
• Chemistry - Will The R...
Predicting Products of SR Reactions:
• Predicting Products of...
___________________________________
Double Replacement Reactions:
• Introduction to Double...
Net Ionic Equations:
• Precipitation Reaction...
Writing Chemical Equations From Words:
• How To Write Chemical ...
Solution Stoichiometry:
• Solution Stoichiometry...
Molarity & Dilution Problems:
• Molarity Dilution Prob...
Acid Base Neutralization Reactions:
• Acid Base Neutralizati...
____________________________________
Acid Base Titration Problems:
• Acid Base Titration Pr...
Mixture Problems:
• Mixture Problems
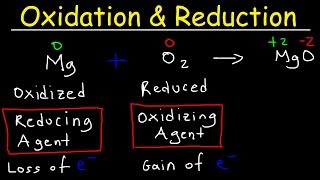
Calculating Oxidation Numbers:
• How To Calculate Oxida...
Oxidation and Reduction Reactions:
• Oxidation and Reductio...
Balancing Redox Reactions:
• Half Reaction Method, ...
Ideal Gas Law Problems:
• Ideal Gas Law Practice...
___________________________________
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections







General Chemistry 1 Review: bit.ly/3DNmZqb
PDF Worksheet - 160 Questions: bit.ly/37SVLn6
Final Exams and Video Playlists: www.video-tutor.net/
You deserve Nobel ❤ I love you ❤
Types of Chemical Reactions
1- Combustion Reaction
• in combustion, something is burning and it releases a lot of heat energy.
• if you see CO2 and water in the product then it's a combustion reaction.
• All "Combustion" reactions are "Redox Reactions".
2- Synthesis\Combination reaction
• A + B = AB
• you simply combine the reactants to make the product.
• If two pure elements combine to form a compound then it's a "Redox Reaction".
• If two small compounds combine to form a large compound then it's probably NOT a "Redox Reaction".
3- Decomposition reaction
• AB = A + B
• the opposite of the Synthesis\Combination reaction: simply breaks down reactants to make the product.
• heat and electricity cause decomposition reactions
• If a compound breaks down into pure elements then it's a "Redox Reaction".
• If a large compound breaks into two small compounds then it's probably NOT a "Redox Reaction".
4- Single replacement reaction
• A + BC = AC + B
• Just as the name says, a single molecule from the same equation replaces another.
• For the most part, metals tend to replace metals and nonmetals tend to replace nonmetals.
• All "Single Replacement" reactions are "Redox Reactions".
5- Double Replacement reaction
• AB + CD = AD + BC
similar to the Single replacement reaction; however, this one is double, and completely switches elements' places up.
• Both of the reactants must be aqueous
• When a double replacement happens between two aqueous solutions and the products all remain aqueous then no reaction took place.
• A "Double Replacement" reaction is NEVER a "Redox reaction"
• Double Replacement reaction types:
- if a double replacement of two aqueous products happens and you find a solid in the product then it's called a "Precipitation Reaction".
- If a double replacement happens between a strong acid and a strong base it will produce water and salt and will be called "Acid-Base Neutralisation Reaction".
- if a double replacement of two aqueous products happens and you find a gas in the product then it's called a "Gas Evolution Reaction".
ـــــــــــــــــــــــــــــــــ
-Redox Reactions: Also called "oxidation-reduction reaction", is any chemical reaction in which the oxidation number of atoms changes.
-Methane(CH4) is also called: Natural gas.
-Almost all ionic compounds are considered salts.
Thank youuu
Lol I'm taking notesfrom you😂
Thank you!
Isn't a base-acid neutralization reaction a redox reaction and also a double replacement reaction but you said double replacement reactions are never redox
Dude thank you
I love you
I love you with a passion you have helped countless sophomores
But I'm a freshman ....
But I'm in 8th grade
I'm in 8th too
@Ahmed Jordan Haha
@Nathanael Donald haha
Here are some timestamps
Synthesis- 2:10
Decomposition- 4:21
Single Replacement- 9:00
Double replacement- 12:08
Combustion- 0:00
Thanks
Thank you man.
Remember kids, not all heroes wear capes and beat villains. Some sit behind a screen and make videos of simplified explanations of hard concepts explained poorly at schools. Some place timestamps on said videos
thank you,you’re a legend
🐐
Synthesis: Putting Together
Decomposition: Breaking Up
Single Replacement: “I’m leaving you for your friend”
Double Replacement: “Wife Swap”
Combustion: “Taylor Swift”
-
-
All Credits to my science teacher
-
The Taylor Swift one: does the same thing over and over again. Gets a boy. They break up. She writes a song.
-
Hope this helps!
@GigaramsYT underrated
omfg i totally thought of a wife-swap analogy to remember double replacement
Damn, I never noticed how all of taylor Swift's songs are all about break up.
😅
@@bivamshukhadka8953 yeah marjorie and soon you'll get better is totally about break up
I seriously think you're the best teacher i've ever had. I really understand when you teach me
the same for me
Fax
True
@@Jxllyyy SHINRA TENSEI
@@soraalmasalmeh5474CHIBAKU TENSEI❤️
What I love about watching your videos is that you get into detail and really explain it in an organized and complex manner without getting too messy. Its perfect.
honestly i've searched so many videos to help me understand this and you finally saved me!
when i read the notes i didn't understand but after watching this tutorial, i clearly understand and can differentiate between different types of reactions. thanks alot
i dont know where i would be in life without this guy
had to watch this for a class and I’m actually surprised at how easy it was to follow, and how much I learned!
th-cam.com/video/ctA5FavFFIA/w-d-xo.html
my teacher is such a good person for finding these good videos.
Professor Organic Chemistry Tutor, thank you for explaining and analyzing different types of Chemical Reactions in AP/General Chemistry. All Chemical Reactions come with different compounds. Breaking down large compounds into small compounds is an excellent way to comprehend Chemical Reactions. This is an error free video/lecture on TH-cam TV with the Organic Chemistry Tutor.
What would I do without you🤔 You have helped me so much. I have watched your videos multiple times. As a gratitude, I watch all the advertisements so you get paid. I wish you were my chem teacher growing up. Your students are the lucky bunch! 🙏🙏🙏🙏🙏
THANK YOU SO MUCH FOR THIS.
I AM IN NEED FOR THIS KIND OF LESSON. ❤
You Helped me through the whole year now I’m watching this in my finals.
thank you again for what u do for the chem community
god bless
I love your videos! Thank you so much for sharing your knowledge! 💓
Being a sophomore, I absolutely couldn't understand ANYTHING. Thanks to your videos, I might actually get it.
Me studying for exam that is 3 hours from now
I salute u
legend HAHA
bro might have just saved my life
it became so interesting to me that I did not notice how 40 minutes passed, thank you very much
Your video has helped me loads because guess what someone wasn't listening to there teacher when they were explaining it lol. Thankk you may you receive more blessings in abundance ❤❤❤
Would you please tell how we can find out when it is aqueous,liquid or solid?
Solubility rules, H2O will typically be liquid unless it's a combustion reaction
Use a solubility chart
it should say it in the question
I really wish I would have known about your channel when I was in college it would have saved me a lot of time and stress. Thank you for your content. It is helping me get into medical school!
Did you get in med ? How’s ur journey so far
did u get into med school?
Did you get in?
Thank you! Helped me a lot.
Really helpful!
Your teaching is stupendous.
Thank you so much this was so helpful!
Best educational youtube channel. No cap
Save time here:
Synthesis
a+b -> ab
Decomposition
ab -> a+b
Single replacement
A+BC -> AC+B
Double Replacement
AB+CD -> AD + BC
Combustion
using heat and basically anything with O2
Good note! Thanks dawg😊
Yes I'm back again teacher. I love you so much
Studying for my chem test that’s in less than thirty minutes 😎
this video has helped me a lot
thank you
Thank you sooo much you ease everything that I thought its soo difficult
BEST TEACHER EVER
I am so stupid that I just cant understand in school but your videos have saved me
You are not stupid. Stop it.
You're really the best thank youuuu 😭😭💗💗💗💗💗💗
I love ur teaching sir
23:50 How do you know when a product is solid vs aqueous/soluble? Just memorize everything?
It's based off of the solubility rules. He has a video on that as well.
I learned so much and you give so much example, that I may be able to learned and to understand the 4 chemical reactions THANK YOU!!!
Good job I love your teaching
Thanks man! We'll be having a quiz in a few minutes. Wish me luck!
how did it go
Thank you so much!!!!
Great teacher
🎉🎉🎉🎉🎉🎉🎉
You're legit saving my ass rn, I have a exam tomorrow and this sir is saving me
TYSM for this one!
This is easier to understand than when my prof teach.
I need help figuring out redox and neutralization reactions
I love you with passion! Thank you dad. I LOVE YOU SO MUCH MWAH
you always save my life GOD BLESS YOU
this si really helpful!! i got 99 on my exam score!!
You a actually the best
Thank you!
thanks for your great detailed explanation
In 37:37, is it also possible to have a combustion reaction there since it produced water and CO2?
thanks alot save me from failing general chemistry
Good job 👏🏼❤️
Thank you man!
Ahh ee we got an exam tmro:)
Thank you!❤️❤️❤️
Thank you for this
Dude.Thank you.
best video ever
Thank you
Thank you!!
you teach me more than my professor
thanks for the video : )
In the decomposition ex. 1 Why does metal oxide has still oxygen, if all the gases will escape to the air?
on 28:39 one of the products isn't Na2SO4 but K2SO4.
Yes! Nobody seems to notice
yes! thank u for pointing out. i thought i just didn't understand my lesson that much lol
Have you even watched the whole video?
@Filip Borin
@@francinemontemayor7428 He corrected it🤦🏽♀️
blud thanks have a test coming up thanks for helping me with this topic
Thank you 🙏
U r amazing thank u bro
Thanks so much ❤
You are too good😭😍💕
Thank you!!!
Your one of the 👌
Thank you!!! :)
May God bless you🙂💗
Thank you 🙏🏽
(Decomposition or analysis) vs (combination or synthesis), I believe analysis was missing at decomposition part
I thought chemistry was easy! I LIED! It's HARD! But this video helped me much! ❤
This guy is better than my chem and physics teacher combined, he must be an angel from Jesus himself
wonderful
In your other video of Predicting the Reaction
You have inverted Synthesis and Decomposition reaction
Pls, I'm confused which one is correct
Just know that synthesis is the coming together of two elements to make a compound or the coming together of two compounds to make a larger compound while decomposition is the breaking down of compound which is the product into elements which is the reactants
Very good
thanks alot
Great!!
I love your videos!!! Now I don’t have to trade nud3s for Chem help back at my college😣 You’re amazing @TheOrganicChemistryTutor ❤
Thanks
Best!
With the simple replacement reaction when do you know when to replace A with B or C ?
Always the first one
I'm a chemistry student from Liberia, how can be a member of your patron platform ?
real hero
Gordon's chem class wya
You must be an undercover harvard teacher fr fr no cap
I get how you do the reactions but not the why
Why does there have to be a reaction in general?
E.g. Na2S + HCl -> NaCl + H2S
Does everything react or do some stay the same?
Fondamental lecture!!!!
Mg + O2 = Mgo...can this be also a combustion reaction? coz a lot of heat and light is given off.
Very informative.
You are a idiot
alpha core an
22:16 why the reaction takes place since Pb is less reactive than Na?
PLEASE SOMEONE ANSWER ME
Why at 8:35 is the top equation not an acid replacement? Doesn’t an acid replacement produce salt and water?