MnO3F is Much More Reactive than Mn2O7! Permanganyl fluoride.
ฝัง
- เผยแพร่เมื่อ 9 พ.ค. 2022
- Suddenly I got the idea to mix potassium permanganate and fluorosulfuric acid to get PERMANGANYL FLUORIDE (MnO3F) and I did it right away!
KMnO4 + 2HSO3F → MnO3F + KSO3F + H2SO4
Permanganyl Fluoride: A Brief History of the Molecule MnO3F and of Those Who Cared For It
d-nb.info/1229250409/34
❤️ 💛 💚
Patreon: / chemicalforce
Bitcoin BTC: 1828WxhTtqohRiQBHgKtdqrmxsGncsjva2
❤️ 💛 💚 - วิทยาศาสตร์และเทคโนโลยี





![ความรักของยักษ์เขียว (ทศกัณฐ์) - เดี่ยว ไออุ่น [OFFICIAL MUSICVIDEO 4K]](http://i.ytimg.com/vi/yCCy-QVHqK8/mqdefault.jpg)


I was looking into making permanganyl halides very recently, actually! The fluoride seems to be the only stable one though (stable being a relative term, obviously), so I couldn't really make any myself due to lack of fluorine compounds. So glad that you could once again satisfy my curiosity with such a quality video! Keep it up man!
Ch2cl2 ssd
You can buy CaF2 from pottery shops super cheap and might as well get a bunch of other stuff to make the shipping worth it
Permanganyl Fluoride is basically an angrier sister of the classic Permanganate Heptoxide, as addition of Fluorine tend to make the oxidizers a little bit more exciting. Great demonstration of both Manganese-based Oxidizers.
If by interesting you mean HF as a byproduct of decomposition, then sure...
Some beautiful colors in these reactions.
Well, I should admit I never heard about the substance.
The result was quite unexpected for me - given that similar substance (perchloryl fluoride, ClO3F), is obviously a hell to handle, but not as touchy as Cl2O7
Hey! you can find some ClO3F in the magic acid video 😀
Magic acid has a quite different connotation in English. Thanks for another great and fascinating video!
@@bobsmith6079 even better is making magic acid and filling a container with it then putting Teflon on top of the container. And linking it to another bottle containing super hot potassium hydroxide dissolved in a 100proof vodka and you use a string to pull the Teflon from between them from 40 ft away and watch it erupt while on shrooms
The cinematography on this one was incredible. I was absolutely riveted by the colours and the reactivity.
That green and purple smoke looked like the nastiest reaction I could imagine lol. So reminiscent of putrefaction and decay! And the ethanol looked like it was burning with quite some heat. What beautiful reactions.
This is still one on the most fascinating channels on TH-cam. Also one of the scariest 😅
🤭
I had never heard of MnO3F! Being a geochemist I feel like I miss out on the more exotic compounds.
Lovely video, you chose a great set of reactions and filmed them beautifully.
Excellent video, thank you!
The slow-mo and the music match so perfectly! Good work!
WOW!!! Incredibly Beautiful!!!
Fluorine stuff is scary. Some time ago I was sealing some refrigeration thingie filled with 1,1,1,2-tetrafluoroethane. I was using molten tin alloy, and open flame was used to heat the copper tube. When the flame came into contact with this "non-flammable gas" it produced some reactive fluorine species, and that was brought into my eyes by wind. The feeling was like someone smacking my eyeballs with a rubber band. And yes, I was wearing safety glasses, didn't expect *that* however, (they were not gas-proof). Fortunately it made me turn rapidly, so I was exposed for a fraction of a second. Don't recommend it to anyone.
Carbonyl fluoride formation?
@@Trifosgene Could be, as it's one of a few possible thermal decomposition byproduct. It's NASTY.
you are very brave chemicalforce. thank you very much for the video and the quick chemistry class ;)
So incredibly beautiful, this is something you wont see everyday, although now we can because its on video! The colors are crazy, and the slowmo of the ethanol drops is absolutely incredible!
First time I’ve seen this. One of the reasons I love this channel
You are a legend man! That looked otherworldly.
You got me. Never thought this compound was even possible, let alone be stable enough to see it in action.
Thank you for doing the reactions I want to see, but also want to be as far away from as possible!
Thanks for sharing buddy
Однозначний лайк. Дуже класний контент. Браво. Дуже гарна якість зйомки. Як кажуть хочу ще.
Although I have absolutely no clue what is going on in these videos, I love watching the reactions. Very well produced, keep them coming.
I do appreciate this channel showing off the more exotic oxidizers, not least because some of them make much more interesting colors than a common flame.
MnO3F??? Yeahhhh… again something new by Felix!
Thanks man! Always improving and new stuffffffff. Enjoyed it very much!
De fato não conhecia este reagente!
Muito bom!
👏👏👏👏👏
I know it very well. Salts of Perfluorosulfonic acid and perfluoroalkylsulfonates are used extensively in the semiconductor industry as surfactants and superacids for photoacid generators.
The slow motions are absolutely beautiful !
It was like watching a volcano erupt on an exotic island :)
Wow very cool. Great colors too
Sick camera work
you madman love it
So good!!!!👍👍👍
My boy Samarium getting some love
happy 120k !!!
so many cool colors
even though it's a video and nothing can hurt me, I'm still holding my breath at the sight of those fumes
Yoo! I love your videos! When are you going to do XeF2?(no pressure intended)
MnO3F is quite interesting, but one step back to manganese(VII) oxide: I would like to see Feliks or someone else with proper skills and equipment to finally isolate PURE Mn2O7 and explore its properties. Otherwise you only ever get to see the crude reaction mixture between conc. H2SO4 and KMnO4 as the normal case. Because of the protonation/condensation/dehydration equilibrium reactions taking place, this reaction mixture most likely consists of excess H2SO4 or KMnO4, the byproducts KHSO4 and [H3O]+[HSO4]- and finally the target compound Mn2O7 accompanied by unknown proportions of permanganic acid HMnO4 and even permanganyl hydrogensulfate [MnO3]+[HSO4]-. Perhaps the isolation/purification of Mn2O7 from the reaction mixture could be done by vacuum sublimation at low temperature. Isolation of permanganyl salts with the cation [MnO3]+ would even be more interesting and challenging. MnO3F probably isn't such a salt but a molecular compound.
You theoretically could distil it under reduced pressure and temperature. Would be next level risky though. 😲
@@christopherleubner6633 You'd probably be best off extracting it with a freon and evaporating the solvent. Just don't make the mistake I made and use dichloromethane. Stupid, stupid, stupid.....
Amazing: The little drop bounces on the big drop at 2:55
Permanganyl fluoride... Sometimes, you can tell a chemical is going to be volatile and scary just by the name.
Excellent footage!
Why did you select Samarium?
Anybody who knows a young person that's interested in science should share this video with them.
It's a pretty accurate representation of what I imagined the Mad Scientist's Chemistry Lab would look like, except Feliks manages to make this entertaining AND educational. :D
Great stuff mate.
Superb videography! A joy to watch. Could we get 60Hz tooooo? :)
Wow crazy chemicals. Cool chemical reactrions
As always top stuff! I am curious what is the most smoke emitting reaction 🤔?
Probably burning white phosphorus creating phosphorus pentoxide. It's used by militaries to make smoke screens officially. Unofficially it's used to burn and poison an enemy. There's also TiCl4, but it's more of a fuming liquid. Also used by militaries to create smoke screens.
5:25 Patrick: Hey, SpongeBob. Wanna see this neat chemical compound? Uh, oh. Are you OK, SpongeBob?
This would make some cool firework/smoke effects though 😎
Love the reactions - The SloMo Guys need to pay you a visit :D
Very interesting compound. What about making some stinky isocyanides in the next video and doing reactions with them
This is something I wanted to see for a long a*s time! Now you've done it makes me wanna salute you! Respect+, homie!
BTW, if you can pls do vids on Peroxyphosphates and split cell electrolysis (I forgot the actual name of that! It envolves electrolysis using a membrane)!! Once again, ❤ your works!!!
Usually called membrane electrolysis.
@@chemistryofquestionablequa6252 Thanks for lettin' me know, fellow nerd! 👍👍
@@heisenbergstayouttamyterri1508 any time. If you're really interested in electrochemistry, check out the Mysteriusbhoice channel. He does some awesome stuff with electrolysis and is really friendly and helpful if you don't understand something or need help.
@@chemistryofquestionablequa6252 Yes I'm aware of him and ScrapScience and watched few of their vids! Extraordinary stuff just like Chemforce! :)
@@heisenbergstayouttamyterri1508 scrapscience is great too!
Genial!
Nice! Now could you do a video on anhydrous HF? There's very little video documentation on it and it would be great if you could show it off!
I guess he will gladly pass on anhydrous HF.
@@ChristianMiersch He made a video on hexafluoroantimonic acid tho...
You should do Picric acid (Tri Nitro Phenol) mixed with PbO2: you will not be disappointed !
this man literally has every single chemical on earth
Ohh nooo SpongeBob!!! Lol amazingly powerful reactions here
i have to wonder, what is your table/fumehood bottom made out of? Ie whats the white flooring you conduct your reactions on?
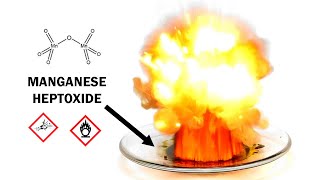
Manganese Heptoxide *I don't wanna exsist nomore!* 💥🔥
Permanganyl fluoride: Even spicier dimanganese heptoxide. Now hydrolyzing to spicy bone-hurting HF.
That ethanol flame looks straight out of a Hollywoord movie special effect, AMAZING! :D
Also, I think you kept saying fluorosulfuric acid instead of fluorosulfonic acid, unless I missheard that?^^
Does this also work when using electrophilic fluorinating agents like NFSI or salts, like AgF for example, which is itself also an oxidizing agent?
It's the same thing, sulfonic = sulfuric (for this one at least.)
Of course I knew that..!!!
Can you make a video where you show the clean up?
You have by far and away the best videos of chemical reactions on the internet. Would you care to comment on your cameras and set up, if not I completely understand and either way I'll be back for more of your fantastic videos whenever you post them. Thanks!
This is the closest you'll ever get to seeing the color octarine.
Green! New and improved! Arsenic-free!
WOW!!!!
Is it time yet for interhalogenic fluorine compound? IF7 would be cool to see or BrF5
Sir what time did you make this video
I add it to my wheaties in the morning
Maybe thats why my toilet paper does that when i use it.. 🤷♀️
Would it be even more violent if you used trifluoromethansulfonic acid? Or would it react with the CF3 group since it’s sorta an organic group?
Basically, it's other chemical name is "Manganese Trioxide Fluoride"
How do you clean and neutralize your fume hood after these experiments
Can you do a video about sodium bismuthate and oxidize manganese 2 to manganese 7 ion?
cool
I wonder what gives the close-hugging smoke/vapor that striated pattern. It looks like the blob has sprouted hair. Laboratory curiosity indeed.
You must have bought the 1000 pack of flint-glass watchglasses!
I wonder what happens if there is a pressure becomes involved.
Why not extracted it with tetrachloromethane?
There are so many cool reactions with fluor compounds. But I still try to avoid working with unstable fluor compounds when possible, cause:
Man, that stuff be nasty!
Yeah, as an amateur i wont ever work with things like mercury or chromium. Fluorine on a whole different level of hell no for me.
If you were to get some of this on your hand... At least you wouldn't have to wait long!
What would happen if you mixed potassium permanganate with fluoroantimonic acid?
I still wanna see H2O2 + LiAlH4
Do borazol
I will like
WOW. Freaking strange reactions you come up with. Genius or a mad man? :D I love your videos: strange, esoteric chemistry and art!
this maybe a stupid question to even say to an intellectual like u coming from me who knows very little to nothing about the periodic table but would u do any reactions using strontium? i don't know if its safe or what but I'm curious what it even is .
try KMNO4 with glycerine
Personally I would not eat that, but I guess we can not all have the same taste.
But will it react with concentrated hydrogen peroxide?
Scary stuff that looks so easy 😲. Especially I dont really like working with stuff involving HF. Interesting though.
Explosion is explosion yet for HF I have huge respect, I guess one of the worst chemicals you can get exposed to if you are not careful enough. Horrible accidents horrible pain and I guess quite many people died even when their hands got exposed to HF.
Interesting though that toothpaste containing NaF is more or less harmless, for obvious reasons.
well and if concentrated HSO3F is comparible to concentrated H2SO4 you have yet another potential source of heat there
I'll rate these compounds a solid Nope out of 10. Wore full body protection and a respirator while watching.
ممكن ssd ch2cl2
I'm curious to try this with triflic or even bis(trifluoromethanesulfonimide) acids. Shouldn't produce HF on hydrolysis but curious about their stability.
I wonder, what would happen, if a premix of EtOH (anhyd.) and HSO3F would be added to KMnO4. Would you expect a more violent reaction?
Ethanol would react with HSO3F to form ethylsulfuric acid and HF: HSO3F + EtOH -> EtOSO3H + HF, so the MnO3F wouldn't form.
@@alexanderkroboth3310 At room temperature, quickly?
@@sebastiand152 yes
@@sebastiand152 Yes, ethanol reacts pretty much the same as water because of the OH group. But you can maybe do that with aceton or some other compound without an OH group.
@@alexanderkroboth3310 Do you know this or is it an assumption?
With acetone, I would expect aldol condensation. At least immediate protonation.
You should isolate the MnO3F.
Pretty, alarming, colours.
the footage along with the editing is ABSOLUTELY amazing, best so far! and check out Jake Tran's latest video, it would be awesome to see this channel grow
How MnO3F is decomposed? I assume the following reaction: MnO3F -> MnO2 + 1/2 O2 + 1/2 F2.
Oxygen is the only element that can bring highest oxidation states to most elements. Oxygen brings nitrogen to +5 oxidation state, where as fluorine even fails to make OF3+. Oxides generally have more thermal stability than fluorides. Oxygen is the king of oxidizers.
F2 seems unlikely - I would expect Mn fluorides, oxides and O2
@@davidbrook7623 I think it will be like the decomposition of nitrogen oxyfluorides. Oxygen is always attached to nitrogen when NOF, NO2F and NOF3 decompose.
Pretty colors. Looks like pure death.
It must be reacting with pretty much anything! Maybe not Teflon..
My man works with chemicals, that most chemists would just refuse to even look at. Respect.
The Green Devil.
It makes HF and you're in the same room?! Please tell me this room is a fume hood and you don't have any neighbors.
Let's some hexane touch it, it will burn.
But cmon, it is not as nearly explosive as Mn207!