Using the Nernst equation | Redox reactions and electrochemistry | Chemistry | Khan Academy
ฝัง
- เผยแพร่เมื่อ 4 พ.ย. 2024
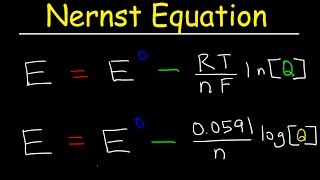
- Using the Nernst equation to calculate the cell potential when concentrations are not standard conditions.
Watch the next lesson: www.khanacadem...
Missed the previous lesson? www.khanacadem...
Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful.
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s Chemistry channel: / channel
Subscribe to Khan Academy: www.youtube.co...








This was extremely helpful. Could you do more chemistry questions based on geology and geosciences in future? Thats the degree I am doing and would be super helpful. Love your way of explaining. Thanks again
Very clear explaination..you are the best teacher i have ever known....big big salute!!!
Great aid in my pursuit of General Chemistry 2. Thanks so much 👍
I'm just lost on why you would add the cathode and anode when the formula is Ecathode - Eanode = Ecell.
Ecell = Ecathode - Eanode when they're both reduction potentials
*If the cathode is reduction potential, while the anode is oxidation potential, you can just add them up (you normally subtract anode because you're trying to turn a given reduction potential to an oxidation potential)
Sir why we use nernst equation to determine non standerd electrode potential?why we not determine electrod potential without nernst equation like Standerd elecytode potential.
Very helpful sir
You like god of chimestry man...
From iraq i'm following you....
You helped me some how God bless you
I hope he could, but he doesn't exist.
When studying corrosion, I have found from many authors (and I teacher I had) that the sign in the equation is (+) not (-) and Q equals to the cations concentration. Could you explain this for me please? Is driving me crazy not knowing which sign should I use in the equation. It depends on what?
Is k and Q calculated in the same way?
E0=Ecathode-Eannode
E0=0.34-0.76
Thank you
Why can't we use nersnt eq for electrolytic cell?
Does anyone knows which program is used for making this video? I mean the whole drawing thing...thank you
Wouldn't Q=(products to the power of their coefficients)/(reactants to the power of their coefficients?)
+Abigail Goff Kind of like you would in equilibria?
+Abigail Goff "Q" is called 'the reaction quotient' and "K" is called 'the equilibrium constant'. They are both calculated in the same way.
Q or K = the ratio of the concentration of the products and the reactants of a reversible reaction.
Second brackets [ ] are used to show concentration(usually molar concentration) of something. Molar concentration=moles/volume.
There is deference between Q and K. Both are ratios of the same things, but calculated at different phases of a reaction. K is during equilibrium. That means, at equilibrium, Q=K
But at other times, QK and this gives the direction of that reaction. If left untouched any reversible reaction will achieve equilibrium over time.
Q(also K) is thermodynamically related to Free Energy, G. G is also related to Electric Potential Difference or Voltage. Hence the video.
+Mostafa Al-Quadir Can I calculate Pressure and Concrentration in the same equation for Q?
+Top 10s The reaction quotient (Q) is a measure of the relative amounts of reactants and products during a chemical reaction at a given point in time. or simply ratio of [product] to [reactant]. If a product/reactant is dissolved in aqueous solution, we use concentrations for all of them. If all reactants/products are gaseous we use partial pressures to calculate Q. Can we use Pressures and concentrations in a single equation to solve Q?
The answer is yes. But we will need to convert Pressure to concentration and vice-versa as we need. We know concentration [ ]=moles/liters =n/V. We also know PV = nRT. So, P = CRT. This is the equation for conversion. Hope that answers the question.
Note: We don't include solids & liquids in the calculations, only aqueous and gaseous.
+Mostafa Al-Quadir Do you know if it's a minus or pluss sign in Nernst Equation?
As I know the E° of Zn is -0.76 not +0.76 !
since zn is being oxidized in this case you have to change the + to a -
sorry i mean the - to a +
why do you use log instead of ln
Can we use deltaGnaught=-nFEnaught with nernst ?
Someone please HELP!!
1) Where did 0.0592 come from?
2) Why is “n” 2 and not 4...what if electrons gained from Copper was 3 and electrons lost from Zinc was 1, then what would the new n be?
PLEASE HELP 😇
0.0592 is the value derived by putting the values of constants in the equation.
Cause 2 moles of electrons are transferred in the reaction.
And i didn't understand that 2nd one :)
can u please teach chemical kinetics. plzz
Thank you.........you deserve more than a million subscribers ^_^
Thanks man.. HELPED ME.. Khan academy aka "subscribed" :)
is it K =K(w) for the equilibrium constant?
In some books, the formula given is:
E=E°- (2.303×RT/nf)*ln([oxidation]/[reduction])
Can some one tell me what is the 'f' given in this equation.
+Ravinder singh Bedi "F" is Faraday's constant, 9.648 533 99(24)×104 C mol−1
+Ravinder singh Bedi Faraday's constant
That is Faraday's constant. f=96500 C
Amazing video
isnt it Ln Q instead of log Q?
it's base 10 logarithm. The natural log is taken care of in the 0.0592
You have the ecell wrong, you just added the ered and eox but you are supposed to subtract them. e(cell)=e(red)-e(ox)
+Crunchyape e(ox) is actually -0.76 so it adds up to 1.10v
+Pamadimukkala Kalyan Yeah but that part inst in the video, someone new may find this confusing
+Crunchyape we are generally given reduction potential. the formula you've given need be corrected.
We only subtract when both of the potentials are reduction. e red ( cathode ) - e red ( anode ) is correct. Also, e red ( cathode ) + e ox ( anode ) will give the same value.
Hocam çok iyi anlatıyorsunuz yarın yazılım var iyi bir not alırım inşallah
hi good work
What is -nFE??
how did u get 0.0592 V?
:o
using the gas constant, Faraday's constant and standard temp 25 degrees in the same formula to replace R,T and F in E=Ecell- Rt÷nf*lnQ leaving E =Ecell- 0.059÷n*logQ do all the necessary transposition
(8.3145*298K)/96485 does not equal 0.059.. what am i missing?
its a different equation, the one with 0,059 is used when the temperature is 25C the other one with RT/nF is used when the temperature is different than 25C
@@wooze85 Your missing 2.303, multiply with the answer and u get 0.059 :)
the product of gas constant and 298 kelvin and division by faradays number gives you 0.059.
this tutorial' standard values (+/-) are incorrect,please correct it
In school we use a bit of a different formula: E=E(zero)+ 0.0592/n * log((c(ox)/c(red)). And by using that, i get 1,1295 instead of 1,07. I can´t find my mistake and the formula is correct too, according to my textbook. could someone help me out?
firstly,the number of significant figures is wrong,and it should be - not +,you just mistook the sign.Hope you have found it out.
hi
Can somebody help me?
When I use ln(10/1) in the main formula, E=1,04 ; why do we change log for the ln shown in the previous video? I'm lost D:
I don't know if you still need help.I hope you are already clear about that.Yes,in the previous video,we use ln to calculate cell potential.When T is equal to 298.15 K (RT/F = 0.025693 V), we switch from ln to log (ln Q = 2.3026 log Q) to get 0.0592 in the nernst equation
In=2.303log
Turkcesini yapamiyorum ingilizcesinden anlarim belki dedim #yks
what happens if I get a nagetive answer?
zishan ismam negative potential means you are past the equilibrium point
switch cathode and anode, u have them opposite
uffffff.......
abi çok iyi yaa
sound volum are too low, without earphone i cannot hear.
i freaking hate this class why am i here fml
i sometimes have this feeling, it's weird, like being in a small cage inside a lion enclosure or shark cage, it's kind of fun knowing you're never really in any danger. it's almost exciting, and kind of a satisfying feeling. just the feeling of being glad you're never going to be in that position for real. same with math, we are all done this nightmare of chem, but every once in a while we go back and remember the hell we went through, knowing it's never gonna hurt us again. that was weird, probably did'nt answer the question
you high bruh ?
@@parth488 lol