Introduction to Pressure - Force & Area, Units, Atmospheric Gases, Elevation & Boiling Point
ฝัง
- เผยแพร่เมื่อ 29 ธ.ค. 2024
- This chemistry video tutorial provides a basic introduction to pressure. Pressure is defined as force per unit area. 1 Pascal equals 1 Newton of Force per square meter of area. This tutorial gives you an example of calculating the pressure exerted by a book on the table using the weight force of the book and its cross sectional area. This lecture also discusses the inverse relationship between elevation and atmospheric pressure. As the elevation increases, the atmospheric pressure decreases and the boiling point of water decreases. It's easier to boil water on a mountain since the air pressure is so low but it's harder to boil in a valley that is below sea level. This video also considers the gases that make up the atmosphere. Heavy gases tend to sink down and lighter gases rise. In addition, hot air rises and cold air descends.
Gas Laws Formula Sheet:
bit.ly/3TOMhhb
Pressure & Boiling Point:
• Introduction to Pressu...
Gas Pressure Unit Conversion:
• Gas Pressure Unit Conv...
Manometers & Barometers:
• Manometer Pressure Pro...
Water Height & Mercury Column:
• Height of Water in a B...
Boyle's Law Practice Problems:
• Boyle's Law Practice P...
_________________________________
How Does a Bike Pump Work?
• How Does a Bike Pump W...
Charles Law:
• Charles' Law
Gay Lussac's Law:
• Gay Lussac's Law Pract...
Avogadro's Law:
• Avogadro's law Practic...
Ideal Gas Law Problems:
• Ideal Gas Law Practice...
Combined Gas Law Problems:
• Combined Gas Law Problems
_______________________________
Gas Stoichiometry Problems:
• Gas Stoichiometry Prob...
Molar Mass of a Gas at STP:
• Molar Mass of a Gas at...
Gas Density at STP:
• Gas Density and Molar ...
Dalton's Law of Partial Pressure:
• Dalton's Law of Partia...
Collecting Gas Over Water:
• Collecting Gas Over Wa...
_______________________________
Gas Density of Mixtures:
• Gas Density & Average ...
Average Kinetic Energy of a Gas:
• Average Kinetic Energy...
Graham's Law of Effusion:
• Graham's Law of Effusion
Kinetic Molecular Theory of Gases:
• Kinetic Molecular Theo...
Gas Law Problems Review:
• Gas Law Problems Combi...
_________________________________
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections



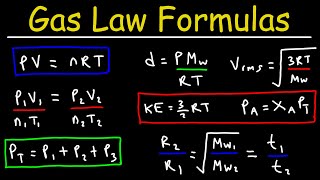





Gas Laws Formula Sheet: bit.ly/3TOMhhb
Final Exams and Video Playlists: www.video-tutor.net/
Full-Length Math & Science Videos: www.patreon.com/mathsciencetutor/collections
Professor Organic Chemistry Tutor, thank you for an excellent Introduction to Pressure, Force, Area, Atmospheric Gases, Elevation and Boiling Point in AP/General Chemistry. Learning these concepts is a good way to understand nature on planet earth. Nature is undefeated on planet earth. This is an error free video/lecture on TH-cam TV with the Organic Chemistry Tutor.
I love the example of a pen tip having more pressure than a finger tip because of the area difference.
thats crazy
At 1:30 the arrow for pressure should point up. When area decreases, the pressure increases. They are inversely related so should point in opposite directions. Either way he explained it correctly.
Very slow voice
I've learned so much more about why things are the way they are from this vid. Much love!
hey your 22 mins are better than my university thanks.
"And your body is like, 'Hey man, stop doing that'"
-Science (1:25)
Thanks for this video! just started learning this topic, really helpful!
This is really helpful.
Very good teacher
This video is so helpful thanks for making it
THANKYOUUU SO MUCHHHHHH !!!!!!
really very helpful .👍👍😊😊☺☺
1000Gigapascle pressure required how much surface area ??
How much bigger an aneroid barometer cell cylinder made with this much of surface area ??
What type of material are appropriate for this work
???
I love the course but ozone is a heavier gas than oxygen, why does it then stay up instead of sinking down
What is work done by aneroid barometer in joules????
How many mechanical barometer provides one kilowatt hour energy forever
yey I'm the hundredth liker, btw your vids are gr8, thanks for teaching
Just wanna ask, is (Pa×m² = N) ?
Force = Area×Pressure (F = A×P)
Dear sir, I have a question-
How can I achieved the pressure that is 10 N/cm square onto a PVC electrical tape by setting a cylindrical tube which is made from material not causing corrosion and also tube will be filled with the appropriate amount of lead shot, to assure the desired pressure.
Please tell me the dimensions of the cylinder and the weight of the lead shot.
I have exam tire and I'm starting it now at 12:20
Tomorrow not tire
That Greta and everything but where did 9.8 come form ?
That's the gravitational acceleration of Earth
9.81 if you want to be specific
How did you get 9.8
Thats the gravitational constant on earth
It's constant of gravity which is either (9.8 or 10)
Btw i cant understand isnt force= mass × acceleration .......weight = mass × gravitional force
Weight is force acting on a body due to gravity and its measured in (newtons).
How the pressure will be 612.5
How it will come
Also 0.7 0.4 . How to calculate
1 atm=101.3kpa not pa
Jai Shri ram❤
Explain
Hindi
i m the 334th liker!!
deez nuts
NickRodriguez 898969 hahah got em
Ha one like only
W